What Produces Heat Is Called?
Heat is a form of energy that manifests itself in our day-to-day lives in many ways. From the warmth of sunshine to the red glow of fire, heat plays an integral role in various natural processes and technologies that power our modern world. This article will provide an overview of the different mechanisms that produce heat energy.
Definition of Heat
Heat is the transfer of thermal energy between substances due to a temperature difference. On a molecular level, heat arises from the kinetic energy of atoms and molecules. As temperature increases, atoms and molecules vibrate and move faster, carrying more kinetic energy. This increased molecular motion corresponds to an increase in thermal energy and temperature.
The kinetic theory of gases explains heat on a molecular level. It states that gases consist of tiny particles (atoms or molecules) that are in constant random motion. The temperature of a gas reflects the average kinetic energy of its particles. As a substance gains thermal energy, its particles vibrate and move faster, causing the temperature to rise. Heat flows spontaneously from high-temperature to low-temperature objects because faster moving particles collide with and transfer kinetic energy to slower ones, increasing their energy and temperature.
On an atomic level, heat causes the atoms within a substance to vibrate. As the atoms and molecules vibrate faster, they bump into each other more often, transferring kinetic energy. This increase in atomic and molecular motion corresponds directly to a rise in the thermal energy and temperature of the substance. In summary, heat arises from the kinetic energy of moving atoms and molecules.
Heat Transfer
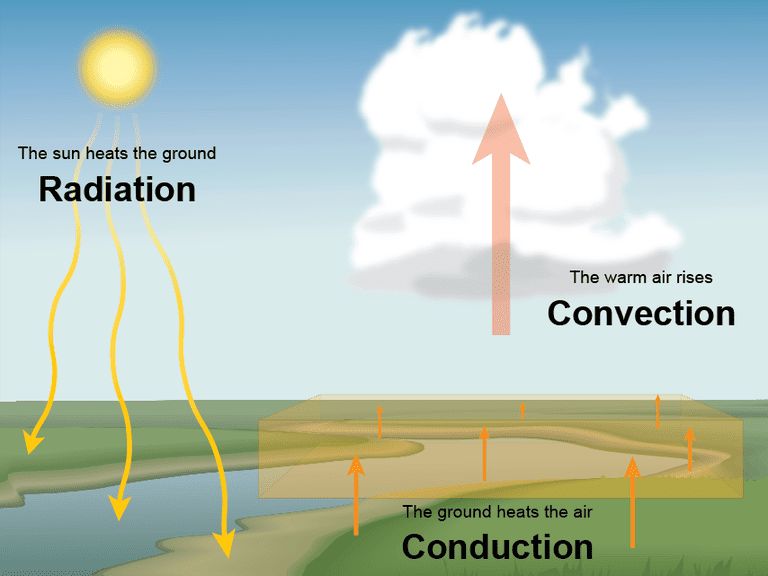
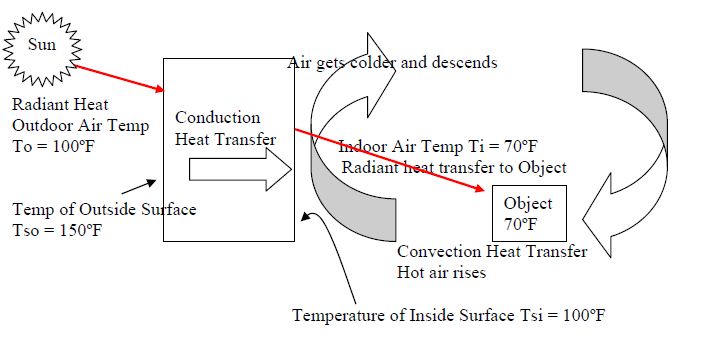
Heat transfer refers to the exchange of thermal energy between physical systems. There are three main mechanisms of heat transfer:
- Conduction – The transfer of heat between substances that are in direct contact with each other. It involves the transfer of kinetic energy between molecules and electrons without any motion of the material as a whole. Metals are good conductors of heat.
- Convection – The transfer of heat by the movement of fluids. It involves the transfer of thermal energy between a surface and a liquid or gas in motion. As the fluid moves, heated particles transfer their thermal energy to cooler neighboring particles. Convection plays a major role in weather patterns and ocean currents.
- Radiation – The transfer of heat via electromagnetic waves directly between molecules or objects. No direct contact is required. An example is the warmth from the sun reaching the Earth. All objects emit thermal radiation related to their temperature.
Understanding these mechanisms of heat transfer allows us to engineer solutions for heating and cooling systems, improve energy efficiency, and predict weather patterns.
Exothermic Reactions
Exothermic reactions are chemical reactions that release energy in the form of heat. Some common examples of exothermic chemical reactions that produce heat include:
Combustion reactions: These involve the reaction of a fuel with an oxidizer, such as the burning of wood, coal, oil, or natural gas in the presence of oxygen. The energy released allows us to generate heat and power.
Thermite reactions: When aluminum powder is combined with iron oxide, it produces aluminum oxide and liquid iron. This reaction releases a tremendous amount of heat, reaching temperatures over 4000°F. Thermite reactions are used in welding applications.
Hydration reactions: When water chemically combines with anhydrous salts, oxides, or alkalis, hydration occurs. Calcium oxide hydrating to become calcium hydroxide releases heat. This provides the self-heating in self-heating meals.
Neutralization reactions: When an acid and a base react, the end products are water and a salt. This reaction gives off heat. Ex: hydrochloric acid reacts with sodium hydroxide to form sodium chloride and water.
So in summary, exothermic reactions involve the release of energy as heat. This occurs during many important chemical processes like combustion, hydration, and neutralization.
Combustion
Combustion is a high-temperature exothermic chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized products, heat, and light. The fuels commonly used in combustion include hydrocarbon fuels such as natural gas, propane, gasoline, and diesel.
During combustion of a hydrocarbon fuel, the hydrogen and carbon atoms combines with oxygen to form water and carbon dioxide, releasing heat energy. Overall, the reaction is highly exothermic, meaning it gives off a large amount of heat. The amount of heat released depends on the type of fuel. For example, when 1 gram of gasoline is burned completely, it releases approximately 44 kilojoules of heat whereas 1 gram of hydrogen gas releases 142 kilojoules of heat when combusted.
The large amount of heat released makes combustion extremely useful for generating energy. Most power generation in the modern world relies on some form of combustion, whether it is the internal combustion of gasoline and diesel engines or the burning of coal, natural gas, or biomass in power plants. The heat released during combustion gets converted into mechanical work and electricity.
Combustion also has many household applications for heating, including furnaces, water heaters, stoves, fireplaces etc. The heat produced by controlled burning of fuels is used for cooking, space heating and hot water production. Thus, combustion of fuels is a key process for generating heat energy for both small and large scale uses.
Friction
Friction is a force that occurs when two surfaces slide against each other. The friction force converts the mechanical energy of motion into thermal energy, or heat. There are a few reasons why friction generates heat:
- When two surfaces rub against each other, the irregularities of the surfaces get pressed together and slide over one another. This deformation and sliding at the microscopic level generates heat.
- The adhesive forces between the two surfaces resist sliding motion between them. Overcoming these adhesive forces requires energy which gets dissipated as heat.
- Any vibrations or sound energy produced as the surfaces interact also dissipates energy that turns into heat.
The amount of heat generated by friction depends on factors like the roughness and hardness of the surfaces, the force pressing the surfaces together, and the speed of sliding. Rougher, softer surfaces that have large contact forces and higher sliding speeds will convert more of the mechanical energy into heat.
Some examples of heat generation by friction include: brakes heating up from friction with the wheels, rubbing hands together to warm them up, drilling into wood causing the drill bit to heat up, and meteorites burning up due to friction with Earth’s atmosphere.
Electrical Current
Electrical currents that flow through a conductor, such as a wire, produce heat due to the resistance of the conductor. Resistance is a measure of how difficult it is for electrons to flow through a material. As electrons flow through a resistive material, they collide with atoms or molecules in the conductor, losing some of their energy in the form of heat.
The relationship between electrical current, resistance, and heat is described quantitatively by Joule’s first law, also known as Joule’s law of heating. This law states that the amount of heat (Q) generated by an electrical current is proportional to the square of the current (I) flowing through a conductor multiplied by the resistance (R) of the conductor over a given time period (t):
Q = I2Rt
So the greater the current flowing through a resistive conductor, the greater the heat generated. Electrical appliances like stoves, toasters, and incandescent light bulbs rely on this effect to produce heat for cooking, heating, or lighting. The resistance wire inside these appliances gets hot enough to glow and produce thermal radiation.
Electrical transmission and power lines also produce heat due to resistance in the wires. This can become a major concern at high currents and must be accounted for to prevent wiring damage or fire hazards.
Nuclear Fission/Fusion
Nuclear fission and fusion are nuclear reactions that produce enormous amounts of energy in the form of heat. Nuclear fission occurs when a heavy radioactive element like uranium or plutonium splits into two or more lighter nuclei, releasing neutrons, photons, and a tremendous amount of energy in the process. Fission reactions are used to generate electricity in nuclear power plants. On the other hand, nuclear fusion takes place when two light atomic nuclei fuse together to form a heavier nucleus, releasing energy. The sun produces energy via nuclear fusion, fusing hydrogen atoms into helium. Both fission and fusion produce heat through the conversion of mass into energy, as described by Einstein’s famous equation E=mc2. Even a small amount of mass conversion releases huge amounts of energy. Controlled nuclear fission and hypothesized fusion reactors are capable of producing heat energy on scales rivaling the heat output of the sun itself.
Metabolic Reactions
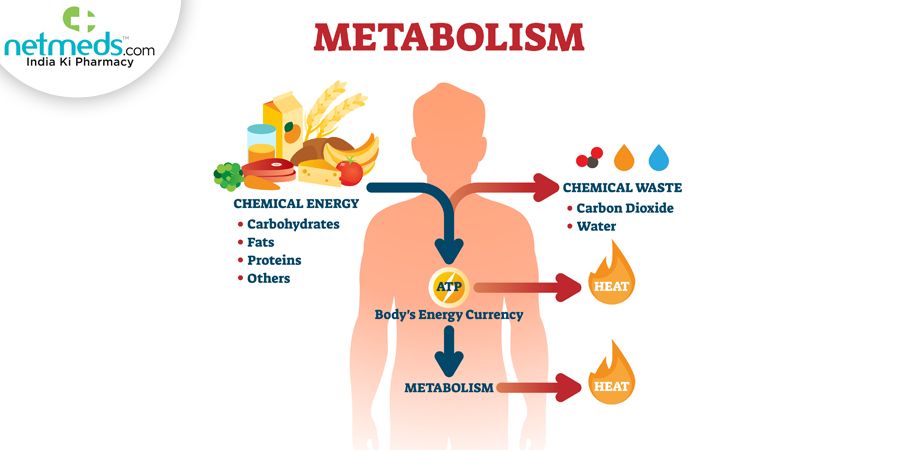
The heat generated by animals comes from the metabolic reactions constantly taking place in our cells. Metabolism refers to all the chemical reactions involved in maintaining the living state of an organism. These reactions allow animals to grow, reproduce, repair damage, and respond to their environments.
A key process is cellular respiration, where cells break down nutrient molecules like glucose to generate ATP, the molecule that provides energy to power metabolic reactions. This process requires oxygen and produces carbon dioxide and water as byproducts. Importantly, as the chemical bonds in glucose are broken, energy is released in the form of heat.
The amount of heat generated depends on the animal’s metabolic rate. Smaller animals tend to have higher mass-specific metabolic rates. This helps them generate more heat relative to their body size to maintain a constant body temperature. When it’s cold, animals can increase their metabolic rate through processes like shivering which contract muscles to produce heat through motion.
So in summary, the heat required to maintain an animal’s body temperature is a byproduct of the metabolic reactions occurring inside its cells. The rate of these reactions controls the amount of heat released.
Conclusion
There are a variety of ways that heat can be produced. Substances or processes that produce heat are referred to as exothermic. Some of the main exothermic processes that were discussed include combustion reactions like burning fuel, friction from mechanical energy converting to thermal energy, nuclear reactions releasing energy, and metabolic reactions in living organisms. Electricity flowing through resistive elements can also generate heat through resistive heating. Overall, whenever energy is released in the form of thermal energy or the kinetic energy of atoms and molecules increases, the process is exothermic and heat is produced. The term for a process or substance that releases energy in the form of heat is exothermic.