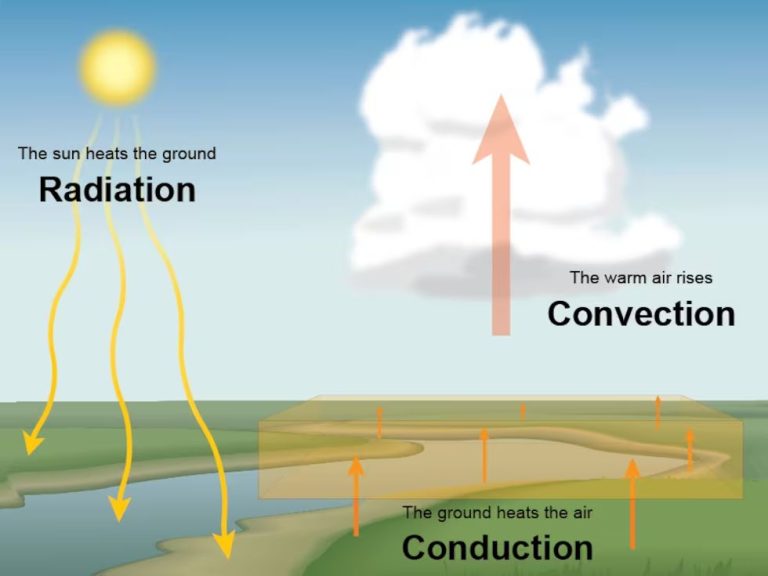
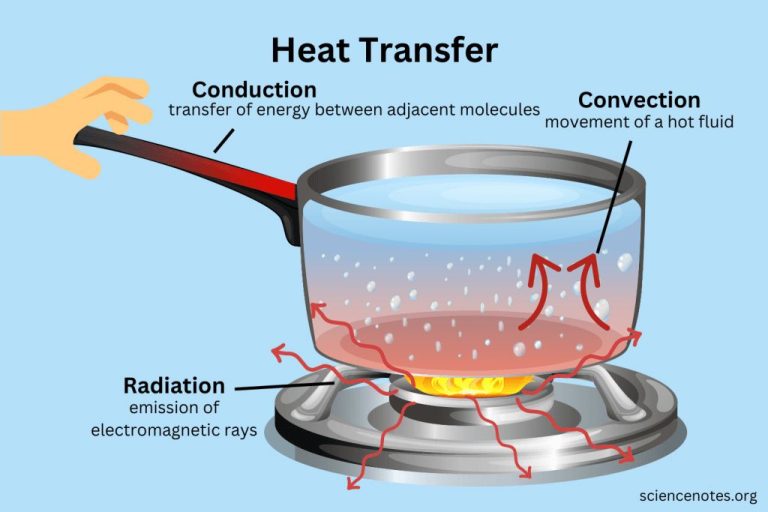
What Is Heat Energy Converted To?
Heat energy, also known as thermal energy, is the energy transferred between two systems or within a single system due to a temperature difference. Heat energy can be converted into other forms of energy through various processes.
There are several types of energy that heat can be converted into, including mechanical energy, electrical energy, chemical energy, light energy, sound energy, nuclear energy, potential energy, and kinetic energy. The conversion of heat into these other energy forms involves the use of heat engines and various thermodynamic processes.
Mechanical Energy
Heat energy can be converted to mechanical energy through engines and turbines. For example, car engines convert the chemical energy from burning fuel into heat energy which pushes on the pistons, creating mechanical energy that turns the wheels. Steam turbines also utilize heat energy from high-pressure steam to spin turbine blades and generate mechanical power.
Other heat engines like Stirling engines can use an external heat source like concentrated sunlight or a flame to heat up and expand a gas which moves a piston to produce mechanical energy. The mechanical energy from heat engines and turbines is used widely to perform physical work like powering vehicles, generators, factory machinery, and more.
Electrical Energy
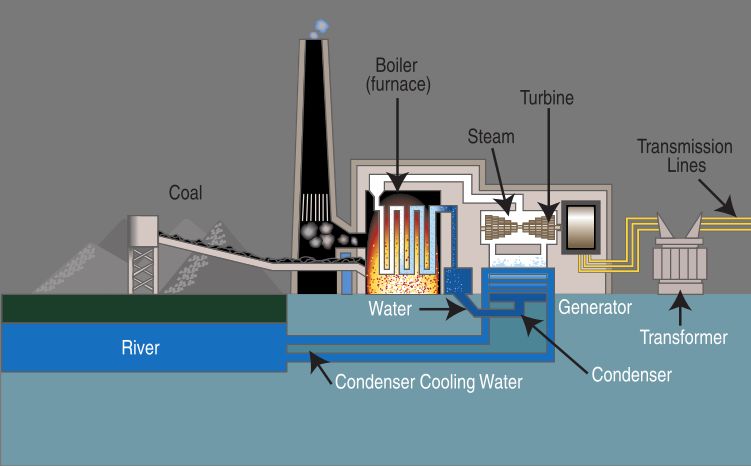
One of the most common ways that heat energy is converted is into electrical energy. This conversion takes place in power plants and generators around the world. The process involves using heat to boil water, which creates steam. This steam is then piped at high pressure into turbines, causing them to spin rapidly. These spinning turbines turn electromagnets within a generator, producing an electric current through electromagnetic induction.
In this way, the initial heat energy from burning fossil fuels like coal, natural gas, or biomass is converted into the motion energy of the spinning turbines. This rotational kinetic energy allows generators to produce electricity that can then be distributed through power grids for use in homes, businesses, and industry. So without the original input of heat, we would not be able to generate the vast amounts of electrical power that modern civilization depends on.
Chemical Energy
Heat drives many important chemical reactions and phase changes. When heat is applied to a chemical system, it can trigger chemical reactions by giving molecules enough energy to rearrange their atomic structures into more stable configurations. The amount of heat absorbed or released depends on the specific change in chemical potential energy during the reaction.
Exothermic chemical reactions release heat. For example, combustion reactions that burn fuels like wood, coal, oil and natural gas produce high amounts of heat energy. The oxidation process strips electrons from the fuel molecules, forming new bonds that result in more stable, lower energy products like carbon dioxide and water. This releases the excess energy as heat. Other exothermic reactions like metal oxidation and the thermal decomposition of compounds also give off heat.
In contrast, endothermic reactions absorb heat. They require an input of energy to break bonds in the reactant molecules before new bonds can form in the products. A common example is the industrial production of ammonia, which uses heat to drive the reaction between nitrogen and hydrogen gases. The absorption of heat causes the reaction to proceed toward products under the right conditions.
Light Energy
When objects get hot enough, the atoms and molecules gain energy and begin to vibrate faster. Eventually, the vibrations cause electrons to jump to higher energy levels. When the electrons fall back down to their normal levels, they emit photons or particles of light. This effect causes very hot objects to glow and give off visible light.
A classic example is a fire, which emits a reddish-orange glow as the wood and charcoal particles heat up. The hotter the fire burns, the brighter it glows. An incandescent light bulb operates on a similar principle. When the tungsten filament inside is heated by an electrical current, it glows white hot, producing visible light.
So in summary, heat and light are directly connected – high temperatures provide energy to atoms which causes them to release photons. This conversion of heat energy into light allows many common light sources, like light bulbs and flames, to brighten our world.
Sound Energy
When heat energy is converted into sound energy, it causes vibrations and movement that create sound waves. The greater the heat, the more intense the vibrations become. Some examples of heat being converted into audible sound include:
– The crackling and popping of a fire as the burning wood expands and contracts from the heat.
– The bubbling of boiling water as the liquid gets hotter and small bubbles of steam burst at the surface.
– Sizzling sounds when food is cooking in a hot pan or on a grill as the heat causes moisture in the food to rapidly vibrate.
– Clicking or ticking sounds from metal and other materials expanding and contracting as they are heated.
In each of these examples, the thermal energy from the heat source causes molecules and materials to vibrate, which creates pressure waves that travel through the air. Our ears detect these sound waves, allowing us to hear the audible signs of heat being converted into acoustic energy.
Nuclear Energy
Nuclear energy is produced through fission reactions, where atoms of radioactive elements like uranium or plutonium are split into smaller atoms. This process releases a large amount of heat energy. Nuclear power plants use this heat energy to boil water into steam that spins a turbine to generate electricity.
In nuclear fission, a neutron collides with a radioactive element like uranium-235, splitting it into two new atoms and releasing multiple neutrons. These neutrons then collide with other uranium atoms, causing a chain reaction where atomic nuclei continue to split and release energy. The energy released is in the form of kinetic energy of the fission products and neutrons, which is converted to thermal energy as these particles collide with nearby atoms and slow down.
This thermal energy is used to heat water, producing steam to run turbines. The steam spins large magnets within coils of wire to generate electricity. Nuclear power plants provide constant power, operating 24/7, in contrast to renewable energy sources like solar and wind which fluctuate based on environmental conditions. Nuclear energy currently provides about 10% of the world’s electricity.
Potential Energy
When thermal energy is used to heat up a substance, some of that energy can be stored as potential energy. This is because heating increases the internal energy of the molecules and atoms that make up that substance.
For example, when water is heated, the increased kinetic energy of the water molecules is a form of potential energy. The faster motion of the heated water molecules means they can do more work compared to unheated water molecules. This stored energy that results from heating the water is a type of potential energy.
In general, heated substances contain more internal energy in the form of molecular motion. This increased molecular motion allows the heated substance to do more work through expansion or phase changes. Therefore, thermal energy that leads to increased molecular motion can be considered converted into potential energy.
Kinetic Energy
When heat energy is added to matter, it causes the molecules and atoms to move and vibrate faster as their thermal energy increases. This intensified molecular motion translates to greater kinetic energy. Kinetic energy is energy associated with movement and velocity. As the particles gain speed and momentum from the added heat, the kinetic energy rises.
We see this kinetic energy boost in action when heating air. As air is warmed, the gaseous particles begin vibrating and moving more rapidly. This faster particle motion manifests as a breeze or wind gaining speed and force. The greater velocity of the heated air molecules results in higher kinetic energy.
So heat speeds up particle motion and increases the kinetic energy of matter. This is observed in warmer air and wind gaining greater kinetic energy from added thermal energy.
Conclusion
Heat energy can be converted into several different forms of energy that are useful in various systems and applications. The key forms that heat energy regularly converts to are:
- Mechanical energy – Heat converted into mechanical energy powers many engines and machines.
- Electrical energy – Thermal power plants use heat to generate electricity.
- Chemical energy – Heat facilitates chemical reactions and changes.
- Light energy – Heated filaments in light bulbs emit light.
- Sound energy – Heat powers sound production in speakers and instruments.
The ability to convert heat into other types of energy makes it an essential driver of many important energy systems that power our modern society and technologies. Heat conversion enables us to generate electricity, power transportation, and utilize chemical processes among many other applications.
Understanding how to efficiently and effectively convert heat energy into different forms allows us to build and optimize essential systems and innovations in energy production and utilization.