Is Energy Made Out Of Atoms?
Energy and atoms are fundamental concepts in science, but not always well understood. This article explores whether energy is made out of atoms. To answer this question, we first need to define what we mean by energy and atoms.
Energy can be defined as the capacity to do work or produce heat. It exists in many forms such as kinetic energy, potential energy, and thermal energy. Energy allows processes to occur and is conserved according to the law of conservation of energy.
Atoms are the basic building blocks of matter. Atoms consist of a nucleus containing protons and neutrons, surrounded by orbiting electrons. The number of protons determines the atomic number and element type. Atoms combine to form molecules and crystal structures that make up the materials around us.
With energy and atoms defined, we can now examine the relationship between the two concepts. Are atoms indeed the constituents that energy is made of, or is the connection more complex?
Forms of Energy
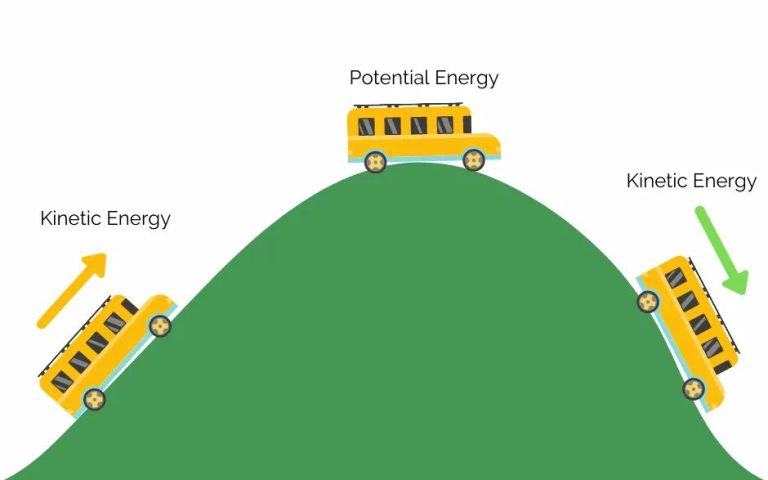
Energy comes in many different forms that can be categorized into two main types – potential energy and kinetic energy. Potential energy is stored energy that has the potential to do work, while kinetic energy is energy in motion. The main forms of energy include:
- Kinetic Energy – the energy of motion that a moving object possesses. Examples include the motion of waves, the motion of electrons, the motion of atoms, and macroscopic mechanical motion.
- Potential Energy – stored energy that an object possesses due to its position or chemical composition. Types include gravitational potential energy from an object’s height, elastic potential energy from deformation of an elastic object, and chemical potential energy from the breaking and formation of chemical bonds.
- Thermal Energy – the kinetic energy associated with the random motion of atoms and molecules. Heat is the transfer of thermal energy between objects of different temperatures.
- Nuclear Energy – the energy stored within the nucleus of an atom, released when a heavy nucleus splits (fission) or when nuclei combine (fusion). Nuclear power plants split atoms to boil water into steam that spins turbines.
- Electrical Energy – energy from the organized motion of charges, such as electrons moving through a wire. Electricity is a common form used in technology and appliances.
- Radiant Energy – energy that travels by electromagnetic waves or photons, such as visible light, radio waves, x-rays, and gamma rays. Sunlight is a common radiant energy source.
- Chemical Energy – potential energy stored in the bonds between atoms that is released in chemical reactions. Examples include batteries, biomass, petroleum, natural gas, and food.
Understanding the different forms of energy is important for studying physics, chemistry, biology, and other sciences. Energy transformations occur constantly around us, powering everything from vehicles to cells. The law of conservation of energy states that energy can change forms, but it cannot be created or destroyed.
Atoms and Their Structure
Atoms are the basic building blocks of all matter. Atoms consist of three fundamental subatomic particles:
- Protons – Positively charged particles located in the nucleus of the atom. The number of protons defines the atomic number and identity of an element.
- Neutrons – Electrically neutral particles located in the nucleus. Neutrons and protons have nearly the same mass. The number of neutrons defines an isotope of an element.
- Electrons – Negatively charged particles that orbit the nucleus. Electrons are much smaller in mass than protons and neutrons.
The nucleus contains the protons and neutrons, while electrons orbit the nucleus in electron shells. The number of protons normally equals the number of electrons, balancing the positive and negative charge. The number of neutrons can vary among atoms of the same element, creating different isotopes.
The structure and interaction between these subatomic particles gives an atom its physical and chemical properties. The number and arrangement of electrons determine how an atom bonds with other atoms to form molecules and compounds.
The Relationship Between Atoms and Energy
Energy comes from the components and structure of atoms. Atoms are made up of protons, neutrons, and electrons. The protons and neutrons are packed together tightly in the nucleus, which contains nearly all the mass of the atom. The electrons orbit the nucleus in regions called electron shells or energy levels.
The energy in atoms comes from the motion and interactions of these subatomic particles. Electrons have kinetic energy as they orbit the nucleus. The positively charged protons in the nucleus exert electromagnetic force on the negatively charged electrons, resulting in an overall potential energy between the electrons and nucleus. There are also potential energies between the protons and neutrons in the nucleus.
These kinetic and potential energies of subatomic particles can be converted into other forms of energy. Nuclear energy comes from changes in the nucleus, such as nuclear decay, fusion, or fission. In nuclear decay, the nucleus releases energy as radiation. In fusion, two nuclei are joined to form a heavier nucleus, converting mass into energy. In fission, a heavy nucleus splits into two lighter nuclei, also releasing energy.
Atomic energy comes from changes in the electron configuration of atoms through chemical reactions or ionization. Atoms form molecules and compounds by sharing or transferring valence electrons, creating chemical bonds that store potential energy. This chemical energy can be released in chemical reactions. Ionization removes electrons from atoms, requiring energy input. But excited ions can later release energy as they decay to lower energy states.
In short, the components of the atom and their interactions result in kinetic and potential energy that can be utilized for nuclear, atomic, chemical, and other energy applications.
Matter vs. Energy
Matter and energy are fundamentally different things. Matter is made up of atoms and molecules that have mass and take up space. Atoms stick together to make up the objects we see and interact with on a daily basis, like a chair, a tree, or even our own bodies.
In contrast, energy does not have mass or take up space. Energy is a property of matter that can be transferred between objects or transformed into different forms. Some examples of energy include kinetic energy, potential energy, heat, and light. Energy allows matter to move, change, or exert a force.
Matter is made up of atoms. Atoms are the basic building blocks that make up all matter in the universe. Atoms are extremely small and are made up of even smaller subatomic particles like protons, neutrons, and electrons.
Energy, on the other hand, is not made up of atoms. Energy is a property of matter. All matter has energy stored within the bonds between its atoms. This energy can be converted between different forms like chemical, thermal, kinetic, potential, etc., but the energy itself is not made up of tangible particles like atoms.
So in summary, matter is composed of atoms while energy is a property that atoms and matter can possess and transfer between different objects and systems.
Converting Between Matter and Energy
Perhaps the most profound relationship between matter and energy is the concept of mass-energy equivalence, which states that mass and energy are interchangeable. This is encapsulated in Einstein’s famous equation E=mc2, where E is energy, m is mass, and c is the speed of light in a vacuum. This equation shows that a small amount of mass can be converted into a tremendous amount of energy.
This mass-energy relationship is dramatically demonstrated in nuclear reactions like nuclear fission or fusion. In these reactions, a small amount of mass from atomic nuclei is converted into massive amounts of energy in the form of heat and radiation. For example, in nuclear fission, the nucleus of a heavy element like uranium splits into smaller nuclei, releasing energy in the process. Nuclear fusion works in reverse – smaller nuclei fuse together into larger nuclei, again converting some mass into energy.
Chemical reactions can also convert matter into energy, although to a much smaller degree. This is because chemical bonds hold less energy per unit of mass compared to nuclear forces. In chemical combustion, for example, the chemical energy stored in the bonds of a fuel is released as heat and light energy, as the fuel reacts with oxygen. Even a reaction as simple as burning wood converts the wood’s mass into energy in the form of fire.
The reverse process is also possible – energy can be converted into mass. This occurs all the time in nature, such as when energy is converted into the mass of newly created particles and antiparticles. High energy gamma rays can decay into an electron and its antimatter counterpart, the positron, which have mass. The process of pair production demonstrates that pure energy is able to produce matter.
While interconverting mass and energy requires tremendous forces only found under extreme conditions, the equivalence of mass and energy is a fundamental truth of our universe that springs from Einstein’s famous equation. This mass-energy relationship has profound implications, from the heart of stars to the core of the atom.
Energy Storage in Chemical Bonds
Energy can be stored in the bonds that hold atoms together within molecules. Atoms bond together because they can reach a lower energy state when paired up compared to existing separately. When two atoms bond, their electrons shift around into more stable positions, releasing a bit of energy in the process.
This energy released during bonding gets stored in the molecule’s chemical bonds, sort of like tightening a spring stores elastic potential energy. Then later on, if energy is put into the molecule to break those bonds, the stored energy gets released again. The amount of energy stored depends on the strength and type of chemical bonds.
For example, the molecules in natural gas or petroleum have lots of carbon-hydrogen bonds which store considerable amounts of energy. When these fuels are burned, their molecular bonds break apart, releasing that stored energy which can then be put to use.
The ability to store energy in chemical bonds is what makes fuels like gasoline and batteries like lithium-ion so useful. The energy gets slowly built up as the molecules form, then quickly released when needed to power cars, phones and more.
Kinetic Energy from Atomic Motion
Kinetic energy is the energy associated with motion. Atoms and molecules are always in motion as long as their temperature is above absolute zero. This motion can be described by kinetic theory. Atoms and molecules have a certain velocity and mass, which gives them kinetic energy according to the formula KE=1/2mv^2. The total kinetic energy of all the atoms and molecules in an object is related to its temperature. Higher temperature means the atoms are moving faster on average, and thus have more kinetic energy.
The kinetic energy of atomic and molecular motion gets transferred in collisions between atoms and molecules. This is how thermal energy transfers from one location to another. The kinetic energy also leads to macroscopic mechanical energy and motion in gases and liquids. As the atoms and molecules collide with the walls of a container, their kinetic energy transfers into the bulk material causing expansion or motion. This is how the burning of fuel converts chemical potential energy into mechanical energy of a car engine for example. The random kinetic motion of atoms and molecules drives the bulk mechanical motion of the entire fluid or object.
Potential Energy from Atomic Forces
Potential energy is the stored energy within an object or system due to its configuration or position. At an atomic level, potential energy arises from the electromagnetic forces between atoms and subatomic particles.
Atoms consist of a dense, positively charged nucleus surrounded by a cloud of negatively charged electrons. The oppositely charged protons and electrons exert attractive electromagnetic forces on each other. However, the negatively charged electrons also repel each other due to their like charges.
When atoms bond together to form molecules or crystal structures, the equilibrium positions of the atoms represent a balance of these attractive and repulsive forces. If the atoms or molecules are displaced from their equilibrium positions, the electromagnetic forces induce a restoring force. This ability to perform work as the atoms return to their equilibrium makes up the system’s potential energy.
The strength of the electromagnetic force between atoms and the distance between them determines the potential energy. Greater displacement from the equilibrium position leads to higher potential energy. This stored energy can be harnessed to do work. For example, chemical reactions that break and form atomic bonds release potential energy stored in the original bonds.
Potential energy is a universal concept that applies to systems and interactions at all scales. But at the most fundamental level, the source of potential energy within matter is always the electromagnetic forces between subatomic particles that make up atoms. So all forms of potential energy have their origins in the interactions between atoms.
Conclusion
Throughout this article, we explored the complex relationship between atoms and energy. We learned that while atoms are the basic building blocks of matter, they also contain immense amounts of energy stored within their structures.
The key takeaway is that energy ultimately comes from the components and interactions of atoms. At the atomic level, energy can be classified into different forms like kinetic, potential, chemical, and more based on the motion, forces, and configurations of atoms.
By converting between different arrangements of atoms, energy can also be transformed from one form to another. Chemical reactions, nuclear processes, and even daily human activities rely on tapping into the energy stored in atomic bonds and structures.
So in summary, even though energy seems abstract, it originates from the atoms that make up all matter in the universe. Gaining a better understanding of atoms and their energetic properties is key to harnessing energy for human use.