Is Heat Energy In Motion?
Defining Heat Energy
Heat energy is a form of energy present in all matter that results from the motion of molecules. It is one of the many types of energy that exist in nature, along with chemical energy, nuclear energy, electrical energy and more. Heat is not a substance itself – it is simply the transfer of thermal energy between objects or systems.
On an atomic level, heat arises from the kinetic energy of atoms and molecules in matter. The faster these particles vibrate and move, the more kinetic energy they possess. This atomic motion generates thermal energy that manifests at larger scales as heat. Objects and systems with fast molecular motion have higher internal energy and temperature than areas with slower molecular motion.
Heat flows spontaneously from hotter to colder matter as faster moving particles collide with slower ones, redistributing the kinetic energy. Thermal equilibrium is reached once heat transfer equalizes the average molecular kinetic energy between two objects or systems. The presence of heat indicates that kinetic energy is being transferred between particles as they interact and collide.
Kinetic Energy and Molecular Motion
Heat energy arises from the kinetic energy of molecules. Kinetic energy is the energy of motion. At the molecular level, heat corresponds to the kinetic energy of atoms and molecules within a substance. The hotter a material is, the faster its molecules vibrate and move.
As a substance is heated, its molecules move faster as they gain kinetic energy. Their increased motion and collisions transfer kinetic energy within the material. The higher kinetic energy of rapidly moving particles is observed as higher temperatures. This molecular motion is directly linked to the heat energy of the substance.
The total kinetic energy of all the molecules in a substance determines its thermal energy and temperature. When heat is added to a system, the kinetic energy of its molecules increases. Removing heat reduces their kinetic energy. This change in kinetic energy at the molecular level is the basis of heat energy and temperature.
Understanding this connection between heat and molecular motion is key to explaining many thermodynamic processes and properties. The kinetic molecular theory of heat helped establish that heat is not a substance itself but arises from particle motion. This discovery revolutionized 19th century physics and laid the foundation for thermodynamics.
Thermal Equilibrium
When two objects come into contact with each other, heat energy will flow between them until they reach the same temperature. This state is known as thermal equilibrium. The colder object gains kinetic energy from the hotter object until their average molecular kinetic energies become equal.
For example, when you place an ice cube into a glass of water at room temperature, heat will flow from the warmer water into the colder ice, causing the ice to melt. This continues until the melted water from the ice cube and the surrounding water reach the same temperature – a state of thermal equilibrium. The kinetic energy (temperature) has equalized between the two objects.
Temperature is thus a measure of the average molecular kinetic energy within a substance. Higher temperature indicates faster molecular motion, while lower temperature corresponds to slower molecular motion. Thermal equilibrium is the state where two objects reach the same average kinetic energy based on heat transfer between them.
Heat Transfer
Heat can be transferred in three main ways – conduction, convection, and radiation.
Conduction is the transfer of heat between substances that are in direct contact with each other. Heat flows from the higher temperature substance to the lower temperature one until they reach thermal equilibrium. The rate of conductive heat transfer depends on the temperature difference and the properties of the materials in contact.
Convection is the mode of heat transfer through fluids (liquids and gases). As the fluid is heated, it expands, becomes less dense, and rises. Colder, denser fluid then moves to take its place, creating convection currents. Convection transfers heat through the bulk movement of the warmed fluid. Convection above a hot surface is called natural convection. Forced convection uses fans or pumps to enhance fluid motion and increase heat transfer.
Radiation is the transfer of heat via electromagnetic waves directly between substances without relying on conduction or convection. All objects emit thermal radiation related to their temperature. Thermal energy is transferred between objects when radiation emitted from one is absorbed by the other. Radiative heat can travel through empty space, unlike conductive and convective heat transfer.
Measuring Temperature
Temperature is a quantitative measure of hotness or coldness. There are different temperature scales that are used to measure temperature:
Celsius scale (°C) – The Celsius scale sets 0°C at the freezing point of water and 100°C at the boiling point of water at 1 atm of pressure. It is commonly used in science.
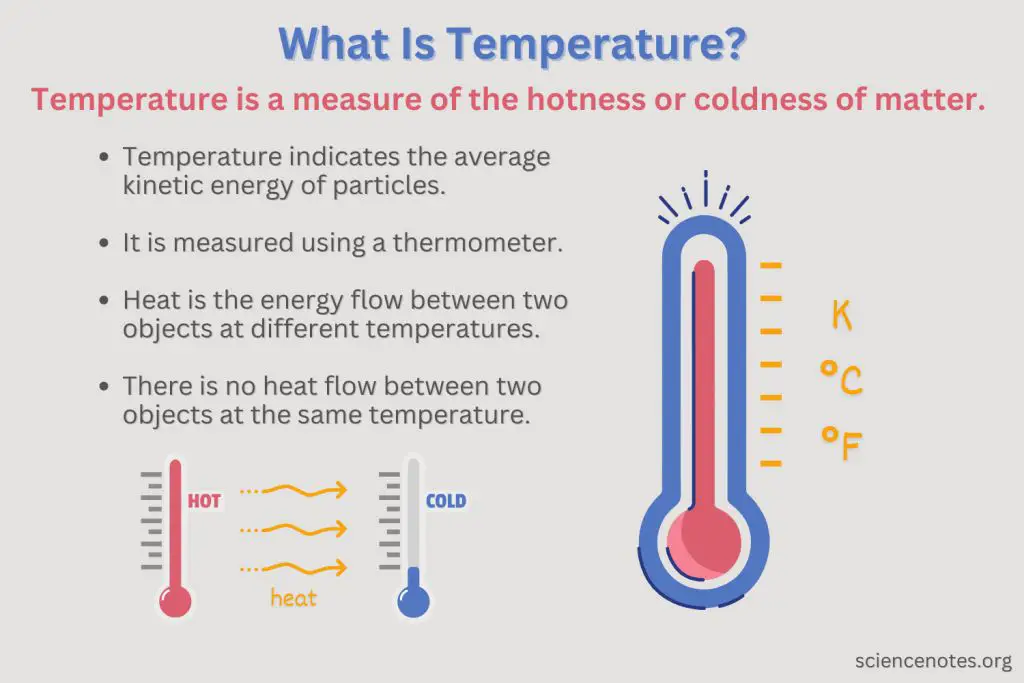
Fahrenheit scale (°F) – The Fahrenheit scale sets 32°F as the freezing point of water and 212°F as the boiling point of water at 1 atm of pressure. It is commonly used in the United States.
Kelvin scale (K) – The Kelvin scale is an absolute scale with 0 K corresponding to absolute zero, the lowest theoretical temperature where molecular motion stops. Water freezes at 273.15 K and boils at 373.15 K. It is commonly used in science.
Understanding the different temperature scales allows us to accurately measure and compare temperatures in different contexts and applications.
Phase Changes
When a substance changes from one state to another, such as from a solid to a liquid or from a liquid to a gas, it absorbs or releases energy. This energy is called latent heat. The latent heat is the amount of energy required or released for 1 gram of a substance to change its state. For example, when water is heated, its temperature increases until it reaches its boiling point. Even though the temperature stops rising, heat continues to be absorbed, breaking the hydrogen bonds between the water molecules, changing it from liquid to gas (water vapor). This latent heat allows the phase change from liquid to gas at a constant temperature.
The high latent heat of vaporization for water enables it to store large amounts of energy. This helps regulate climate and temperature. As water evaporates from lakes and oceans, it absorbs heat from the surroundings. When the water vapor condenses back to a liquid in clouds, it releases heat that warms the air. The large amount of energy needed to change water’s phase makes it effective at moderating temperature changes.
Other common phase changes are melting (solid to liquid) and freezing (liquid to solid). These also involve the absorption or release of latent heat. Just like boiling, even though the temperature stops changing, heat must be continually added to change the physical state from solid to liquid during melting. The reverse occurs during freezing, where heat is released as the liquid becomes a solid again.
Heat Capacity
Heat capacity refers to an object’s ability to store thermal energy. When heat is added to an object, the temperature will increase depending on the object’s heat capacity. Objects with a high heat capacity require more heat energy to increase their temperature compared to objects with a low heat capacity.
On a molecular level, heat capacity relates to the bonds and vibrations between molecules. Stronger molecular bonds and more ways for molecules to vibrate lead to a higher heat capacity. For example, metals tend to have a high heat capacity because the metallic bond allows vibration of electrons over the entire lattice. Water also has a uniquely high heat capacity due to hydrogen bonding between water molecules.
The amount of heat energy required to raise an object’s temperature by 1°C is called its heat capacity. Heat capacity is an intensive property, meaning it depends only on the type of material, not the amount. Units for heat capacity are typically joules per kelvin (J/K) or joules per degree Celsius (J/°C).
Understanding heat capacity allows us to engineer materials and devices to better store thermal energy for applications like thermos bottles, building insulation, and more. It also explains why some objects feel hotter or cooler to the touch compared to others at the same temperature.
First Law of Thermodynamics
The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed—it can only be transferred or transformed. This means that the total energy in a closed system remains constant. This law provides a fundamental connection between thermal energy, mechanical work, and other forms of energy transfer.
According to the first law, when heat Q is added to a system, some combination of the following will occur:
- The internal energy U of the system will increase.
- The system may do mechanical work W on the surroundings.
The first law gives us the equation: ΔU = Q – W
This states that the change in internal energy of the system equals the heat added to the system minus the work done by the system. The law confirms that while energy can change forms, the total amount of energy in a closed system remains fixed. This principle guides the study of thermodynamics and allows us to analyze thermal systems.
Real-World Applications
Heat energy is constantly at work all around us in the real world. Here are some common examples of heat energy in action:
Cooking – The heat from a stovetop or oven allows food to brown, boil, bake, or undergo other chemical changes that make it tastier and more edible.
Internal combustion engines – The exploding fuel inside engines converts chemical energy into heat, which expands gases and pushes pistons, creating motion.
Space heaters and furnaces – These devices convert electricity or fuel into heat through resistance or combustion, warming indoor spaces.
Hot water heaters – Energy sources like electricity or natural gas heat water for domestic and industrial uses.
Chemical reactions – Many important industrial processes rely on heat to drive reactions that produce materials, fuels, plastics, and more.
Phase changes – Heat provides the energy to melt solids into liquids and vaporize liquids into gases.
Metallurgy – Heat is used to shape, anneal, harden, and temper metals in applications from construction to jewelry making.
Incandescent lighting – Traditional bulbs produce light by heating a filament until it glows.
Geothermal energy – The Earth’s internal heat can be harnessed for electricity generation and heating buildings.
Friction – The heat caused by friction allows brakes to stop vehicles and machining processes to shape parts.
Conclusion
In summary, heat is indeed a form of kinetic energy. Heat arises from the random motions of molecules and atoms. As temperature increases, particles move faster and have greater kinetic energy. When two objects at different temperatures come into contact, heat transfers from the hotter to the colder body as the particles interact and exchange energy. This process continues until thermal equilibrium is reached and the objects are at the same temperature. Measuring temperature allows us to quantify the average kinetic energy of particles in a substance. Phase changes also demonstrate the strong link between heat and molecular motion. By adding or removing heat from a system, particles can gain enough energy to change phase from solid to liquid to gas. The concept that heat is the motion of molecules is formalized in the laws of thermodynamics. Overall, the kinetic theory provides a foundational explanation for the nature of heat and its role in our everyday lives.