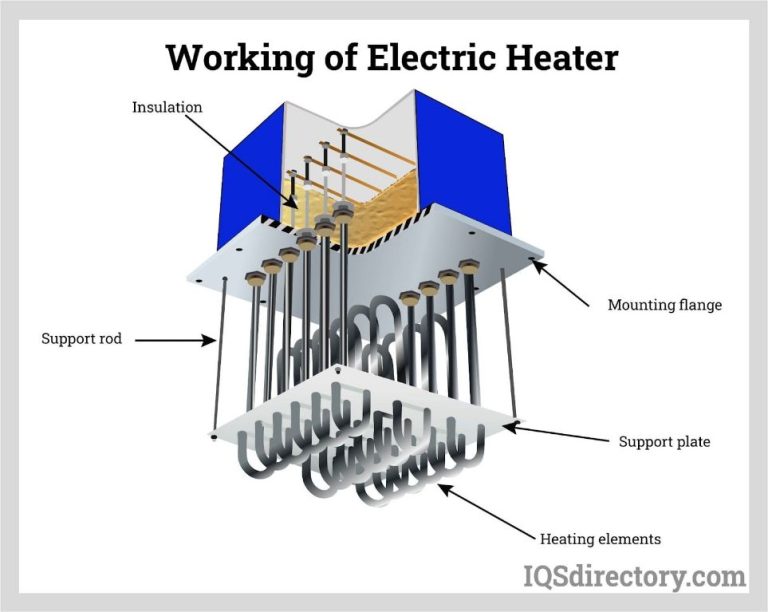
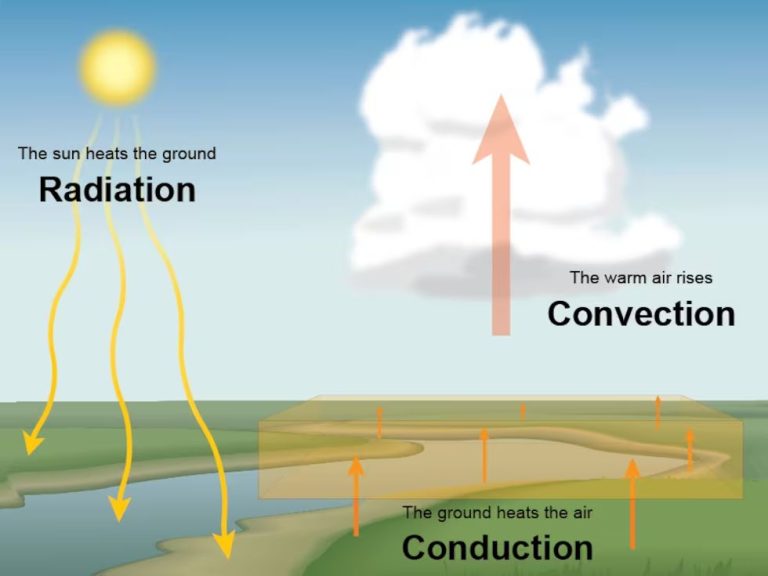
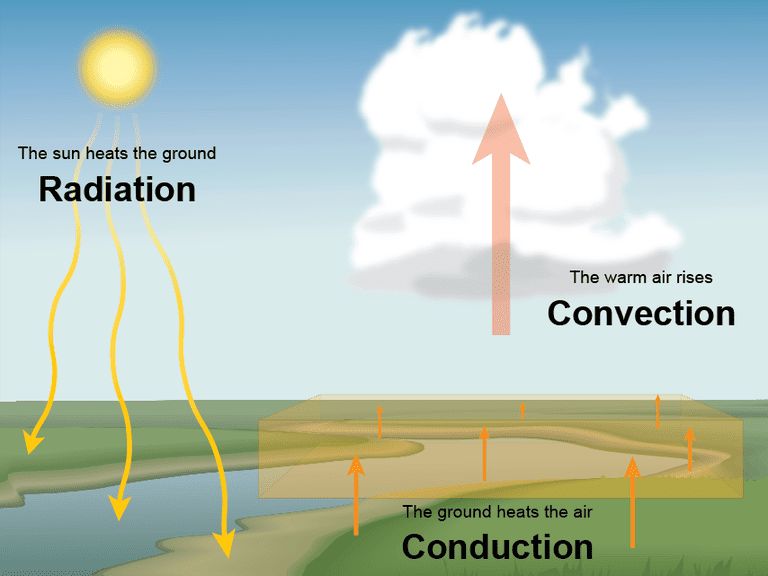
What Are The 3 Characteristics Of Heat Transfer?
Heat transfer is the transfer of thermal energy from one object or system to another due to a temperature difference. There are three main characteristics or mechanisms of heat transfer: conduction, convection, and radiation.
Conduction is the transfer of heat between two objects in direct contact with each other, such as a pot on a stove. The heat from the stove burner is conducted through the bottom of the pot to the water inside it. Metals are good conductors of heat.
Convection is the transfer of heat by the movement of a fluid or gas. As the fluid or gas moves, it carries thermal energy from one place to another. For example, as air currents move around a room, they transfer heat from warm places to cooler places. Convection also occurs in liquids like water.
Radiation is the transfer of heat in the form of electromagnetic waves directly across space. No direct contact or fluid medium is required. An example is the heat from the sun warming the Earth. The sun radiates thermal energy through space, which is absorbed by the Earth.
These three mechanisms allow heat energy to be transferred between objects and locations, enabling many everyday phenomena and technologies.
Conduction
Conduction is one of the three main methods of heat transfer. It occurs when heat energy is transferred through direct contact between materials, without any overall displacement of matter.
In conduction, heat flows from the hotter end to the colder end of an object. This transfer happens as warmer particles vibrate and collide with neighboring particles, transferring some of their energy. The particles receiving the energy begin vibrating faster as they gain heat, and in turn collide with their neighboring particles to pass on the energy. This continues until the thermal energy has been distributed throughout the entire object from the hotter area to the colder area.
Common examples of conduction include:
- Touching a hot stove – the metal quickly conducts heat to your skin.
- Holding an ice cube – heat from your hand is conducted away into the ice, causing it to melt.
- Wrapping your food in aluminum foil – the foil conducts heat away from the food to keep it warm.
Metals are excellent conductors due to their free moving electrons. Other good conductors are graphite and diamond. Insulators like plastic and wood do not conduct heat well because their electrons are tightly bound.
Convection
Convection is the transfer of heat by the movement of fluids. When a fluid like air or water is heated, it expands, becomes less dense, and rises. As the heated fluid rises, cooler fluid moves in to take its place, setting up a circular motion that transfers heat from the bottom to the top. There are two main types of convection – natural convection and forced convection.
In natural convection, fluid movement occurs due to differences in density created by temperature variations in the fluid. For example, when water is heated on a stove, hot water rises to the top while cold water sinks to the bottom, creating a natural circulation. Natural convection also occurs in air – hot air rises while cold air sinks. This explains why hot air balloons are able to rise.
In forced convection, fluid movement is caused by an external source like a pump, fan, or other mechanical means. For example, blowing on hot soup or using a fan to blow hot air are examples of forced convection. Convection ovens use fans to circulate hot air around food more quickly than conventional ovens. Heat sinks on computers use forced convection from fans to dissipate heat away from sensitive components.
Overall, convection transfers heat through the bulk, macroscopic movement of fluids. It is an efficient way to rapidly transfer thermal energy from one place to another. Convection currents play a major role in global air and ocean circulation patterns that distribute heat across the planet.
Radiation
Radiation is the transfer of heat through electromagnetic waves or photons. Unlike conduction and convection, which require matter to transfer heat, radiation can transmit energy across empty space.
All objects constantly emit thermal radiation based on their temperature. Hotter objects emit more intense radiation than colder objects. For example, the sun radiates energy that travels through space to heat Earth. A fire radiates thermal energy that you can feel on your skin from a distance. Different materials radiate at different rates based on their emissivity.
Radiation does not require direct contact between materials or a transport medium like air or water. It works in a vacuum and can travel long distances. In addition to infrared thermal radiation, other types include ultraviolet radiation from the sun, radio waves, microwaves, and visible light.
Some examples of heat transfer by radiation include:
- The sun heating up Earth
- A campfire radiating heat that warms people around it
- An incandescent light bulb converting electrical energy into light and thermal energy
- A greenhouse trapping radiant energy from the sun to increase interior temperature
Radiation is often utilized for heating and cooling systems. Radiant floor heating takes advantage of radiation to warm objects like people and furniture rather than just the air. Solar water heating systems use thermal radiation from the sun to heat water.
Comparing the 3 Types
The three main heat transfer mechanisms—conduction, convection, and radiation—operate in different ways. Here are the key differences between them:
Conduction involves direct contact between materials as heat energy is transferred through the vibrations of atoms, molecules, and electrons. It requires a medium like a solid or fluid. Conduction rate depends on the temperature gradient and the thermal conductivity of the materials involved.
Convection is the heat transfer due to the bulk movement or flow of a fluid. It involves the combined effects of conduction and fluid motion. Convection depends on the temperature gradient and the velocity of flow.
Radiation is the emission and transmission of heat energy through electromagnetic waves or photons. It does not require a medium and can even occur in a vacuum. Radiation depends on the temperature of the surface and its degree of “blackbody” emissivity.
While conduction and convection require some contact or medium to transfer heat, radiation can act at a distance. Conduction and convection transfer heat through matter, but radiation can travel through empty space. Convection relies on bulk fluid motion, while conduction solely depends on molecular activity without overall motion of the material.
Real World Examples
Here are some examples of the three types of heat transfer that we can observe in the real world:
Conduction
When you place your hand on a hot stove, the metal of the stove conducts heat to your hand. The faster heat flows into your hand depends on what material the stove is made of. Metals like cast iron conduct heat quicker than materials like glass or plastic.
In a building, heat conducts through walls, windows, and doors. Insulation placed inside walls slows down this conduction and keeps more heat inside.
Convection
When water heats in a pot, the hotter water at the bottom rises while the cooler water sinks. This cycle allows heat to convectively spread throughout the water.
Air movement in a room, car, or building also shows convection. Hot air rises while cooler air sinks, creating air currents that help distribute heat.
Radiation
On a sunny winter day, you can feel the warmth of the sun’s rays. The sun radiates infrared energy that travels through space to warm your skin.
Fires radiate heat that you can feel from a distance. Incandescent light bulbs also radiate some of their heat.
Applications and Uses
Understanding heat transfer and the three main types – conduction, convection, and radiation – has many important real-world applications and uses. Heat transfer principles are applied in a wide range of engineering and design problems.
Some key applications and uses include:
- Thermodynamics and heat exchangers – Heat transfer calculations are critical in the design of heat exchangers, boilers, condensers, radiators, and other thermodynamic systems.
- HVAC and building design – Architects and HVAC engineers rely on heat transfer knowledge to properly insulate buildings and design efficient heating and cooling systems.
- Electronics cooling – Proper thermal management and cooling is essential in computers and other electronics to prevent overheating. Heat sinks, fans, and other cooling methods rely on conduction and convection.
- Cooking – The heating and cooling of food relies on conduction from hot pans and convection from hot air inside ovens. Microwave ovens use radiation to heat food.
- Aerospace and automobile industry – The hot exterior of re-entering spacecraft or the engine and exhaust of cars utilizes conduction, convection, and radiation heat transfer principles.
- Metallurgy and manufacturing – Controlled heating and cooling is important in metal casting, heat treatment processes, plastic molding, and chemical processing plants.
- Cryogenics – Low temperature physics relies on understanding heat transfer to create very cold cryogenic systems.
- Climate and weather – Heating and cooling of the atmosphere and oceans are governed by heat transfer and thermodynamics.
In summary, heat transfer has many diverse uses and real-world applications in engineering across industries, in the natural sciences, and in everyday life. Properly accounting for conduction, convection, and radiation is key in the design and analysis of many systems and processes.
Related Concepts
Heat transfer is closely related to several other scientific concepts and principles:
- Thermal energy – This is the total kinetic and potential energy of molecules in a substance. Thermal energy flows from high to low temperature as heat is transferred.
- Heat capacity – The heat capacity of a material determines how much heat must be absorbed or lost for its temperature to change. Materials with higher heat capacity require more heat transfer for an equivalent temperature change.
- Thermal conductivity – This describes a material’s ability to conduct heat. Metals generally have high thermal conductivity, while insulators like wood have low conductivity.
- Thermal expansion – Most materials expand when heated and contract when cooled due to heat transfer effects on molecular vibrations and spacing.
- Phase changes – Heat transfer can induce phase changes, like melting ice or boiling water. These phase changes absorb or release significant thermal energy.
- Thermodynamics – The laws of thermodynamics describe fundamental constraints and effects related to heat transfer and conversion between different forms of energy.
An understanding of these related concepts allows for a deeper appreciation of heat transfer principles and how they apply in different situations.
Importance and Impacts
Heat transfer is vitally important to study because it explains how thermal energy moves between objects and locations. Understanding heat transfer mechanisms allows us to properly design technologies, equipment, and infrastructure that rely on the movement of heat for operation. It also helps prevent problems caused by uncontrolled or unintended heat transfer. Some key examples of the importance and impacts of studying heat transfer include:
– Heat management in electronics – Preventing overheating of circuits and components requires knowledge of how heat transfers and dissipates in these systems.
– HVAC system design – HVAC relies on conduction, convection and radiation to heat or cool interior spaces. Proper system design requires in-depth knowledge of heat transfer behavior.
– Cooking and food preparation – Cooking techniques like frying, boiling, baking etc all involve manipulation of heat transfer to prepare food safely and properly.
– Meteorology and weather – Heat transfer in the atmosphere drives weather patterns. Understanding these mechanisms is key to weather prediction.
– Thermal protection and insulation – Insulating materials and design considerations utilize heat transfer knowledge to maintain desired temperatures in extreme conditions.
– Energy generation and transport – Power plants, combustion engines, steam turbines all require careful control of heat transfer reactions to operate efficiently.
By continuing to study heat transfer, engineers and scientists can develop better solutions for managing and utilizing thermal energy across many crucial technological applications and natural processes.
Conclusion
In summary, the three characteristics of heat transfer are conduction, convection, and radiation. Each method transfers thermal energy from one object or system to another in different ways.
Conduction involves direct contact between objects, with energy transferring from hotter to cooler areas. Materials like metals are good conductors. Convection uses the motion of fluids to transfer heat, like hot air rising or water circulating. Radiation works by electromagnetic waves traveling between objects, even through vacuums. No direct contact is needed.
While they function differently at the atomic level, all three follow the basic principle of heat flowing from high to low temperatures. Understanding conduction, convection, and radiation provides insight into many everyday phenomena and technical processes. Whether it’s heating a house, cooking food, or designing a spacecraft, the characteristics of heat transfer come into play.
With knowledge of these fundamental concepts, we can better harness, manage, and apply thermal energy for human needs and innovations.