What Stores Energy In Molecules?
All living organisms require energy to survive and thrive. This energy ultimately comes from the sun through photosynthesis or by consuming other organisms. However, energy from food or sunlight fluctuates, so organisms need a way to store excess energy for later use. This is where energy storage in molecules becomes critical.
The most important molecule for energy storage is adenosine triphosphate, or ATP. ATP acts as the main “energy currency” in cells, safely stockpiling energy until it is needed to power biological processes. Without ATP, organisms would not be able to efficiently store and transport energy. ATP provides a battery-like reservoir of power that can be rapidly tapped as needed, enabling activities ranging from muscle contraction to neurotransmission.
By briefly storing energy in ATP molecules, organisms can accumulate excess energy and then dispatch it on demand. This provides a crucial buffer between variable energy supply and constant energy expenditure. ATP gives cells flexibility in when and how they use energy.
ATP Structure
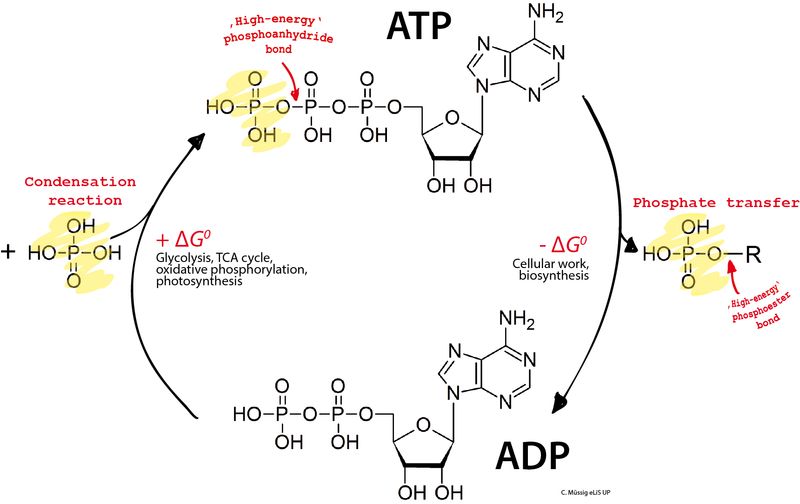
Adenosine triphosphate (ATP) is comprised of an adenosine base attached to a chain of three phosphate groups. The adenosine base contains the nucleoside adenosine bonded to a ribose sugar. Attached at the 5′ carbon on the ribose ring are three sequential phosphate groups linked by high-energy phosphoanhydride bonds.
These phosphate groups are key to ATP’s ability to store and transfer energy. The bonds between the phosphates contain potential energy that can be released when the bonds are broken through hydrolysis. The terminal phosphate group can be detached, turning ATP into adenosine diphosphate (ADP) and harnessing the energy stored in the bond.
How ATP Stores Energy
ATP is able to store energy in its phosphate bonds. It has three phosphate groups that are linked by high-energy covalent bonds. These bonds store energy that can be released when the bonds are broken during ATP hydrolysis. Specifically, the bond between the second and third phosphate groups contains the highest amount of energy. When this bond is broken, energy is released that can be used to power cellular work.
The reason these phosphate bonds can store so much energy is because of the negative charges on the phosphates. Having multiple negatively charged phosphates so close together causes electrostatic repulsion, so it takes energy to overcome this repulsion and form the bonds when ATP is synthesized. Later, when ATP is used, the energy is recouped as the repulsion causes the phosphate groups to break apart.
In summary, ATP acts as the main energy currency of cells because its high-energy phosphate bonds can store energy chemically. Then when ATP is hydrolyzed, these bonds break and release energy that powers many essential cell functions.
ATP Production
ATP is produced through two main biological processes: cellular respiration and photosynthesis. In cellular respiration, ATP is generated in the mitochondria of cells when glucose is broken down. This process, known as glycolysis, extracts energy from glucose to produce ATP. The Krebs cycle and electron transport chain inside mitochondria also produce ATP through oxidation of products from glycolysis. Overall, cellular respiration provides the bulk of ATP generation for animals, who must consume organic molecules like glucose for energy.
In photosynthesis, ATP is produced in chloroplasts of plant cells and some bacteria. When sunlight is absorbed by chlorophyll, it provides energy to convert carbon dioxide and water into glucose, releasing oxygen as a byproduct. This process generates ATP, which plants and photosynthetic bacteria can then use for energy. So while animals mainly produce ATP through respiration, plants and some microbes produce it through photosynthesis. Both pathways generate the universal energy currency of ATP from energy sources available in the environment.
Using ATP for Energy
Once ATP is produced in the cell, it serves as the universal energy currency to power most cellular functions. ATP provides this energy through a process called ATP hydrolysis. During ATP hydrolysis, one of the phosphate bonds on the ATP molecule is broken, releasing energy that can be used to fuel endergonic reactions and biological processes in the cell. Specifically, the bond between the second and third phosphate groups is broken by the addition of a water molecule, leaving ATP with two phosphates to become ADP (adenosine diphosphate). This reaction releases roughly 7.3 kcal/mol of energy.
The energy released allows ATP to transfer phosphate groups to other molecules through a process known as phosphorylation. This gives other compounds energy that enables them to undergo reactions or power biological mechanisms. From muscle contraction to biosynthesis to cell signaling, ATP hydrolysis provides the energy needed to support cellular activities. That’s why ATP is often referred to as the “energy currency” of the cell – it can be “spent” to power critical functions.
ATP Recycling
To understand ATP recycling, we first have to look at what happens when ATP is used for energy. When ATP is used to power cellular reactions, a phosphate group is cleaved off, transforming ATP into ADP (adenosine diphosphate). This releases energy that can be harnessed for biological work.
But we don’t want all of our ATP to eventually turn into ADP, or else we’d run out of our main energy source! This is where ATP recycling comes in. There are enzymes in the cell that can add a phosphate back onto ADP, re-forming ATP. This process is called phosphorylation. Specifically, the enzyme adenylate kinase catalyzes the transfer of a phosphate from another high energy molecule to turn ADP into ATP. This maintains our critical ATP supplies.
By continually recycling ADP back into ATP through phosphorylation, cells can reuse ATP molecules over and over again. This cycling between ATP and ADP allows our cells to conserve and efficiently harness energy from ATP whenever it’s needed.
Other High-Energy Molecules
In addition to ATP, there are other molecules that act as energy carriers and play important roles in metabolism. Two key examples are nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2). These carry electrons and hydrogen atoms that are stripped from nutrients during the process of respiration.
NADH and FADH2 are formed when electrons are transferred to them from substrates like glucose during glycolysis and the citric acid cycle. This gives them a high-energy state. The energy from NADH and FADH2 is then utilized during oxidative phosphorylation to generate ATP. So while ATP is the primary energy currency of cells, NADH and FADH2 also store energy from catabolic pathways and help drive ATP production.
Advantages of Molecular Energy Storage
Storing energy in molecules like ATP offers some key advantages over other forms of energy storage. The compact size of molecules allows the cell to stockpile large amounts of energy in a very small space. ATP and other energy molecules are also highly portable, able to move easily throughout the cell to power reactions wherever energy is needed.
Molecular energy storage is extremely efficient. The bonds within ATP can quickly break to release energy on demand. Cells can then rapidly recycle ADP back into ATP, packing each molecule with energy again and again. Storing energy in chemical bonds also allows gradual release, providing a steady supply of power.
Having a portable, compact, and efficient means to store energy allows cells to survive and thrive. Molecular energy storage gives cells the power they need to maintain homeostasis, grow, move, and perform their many necessary functions. Life would not be possible without the ability of molecules like ATP to efficiently capture, store, and distribute the energy that cells require.
Examples of ATP in Action
ATP powers some of the most essential biological processes in our bodies through the energy released when its phosphate bonds are broken. Two key examples are muscle contraction and nerve impulse transmission.
Muscle contraction occurs when the protein myosin uses the energy from an ATP molecule to pull on the protein actin and shorten muscle fibers. This process allows our muscles to move and generate force. Without the power provided by ATP, our muscles would be unable to contract.
Nerve impulses are transmitted via electrical signaling in the nervous system. This signaling relies on the movement of sodium, potassium, and calcium ions across the neuronal cell membrane. ATP provides the energy to pump these ions against their concentration gradients to create the ion gradients necessary for an impulse to propagate down the length of a neuron. Without ATP, neurons would be unable to transmit signals.
The role of ATP in muscle contraction and nerve signaling highlights its importance in powering some of the most vital processes that allow our bodies to function.
Conclusion
The key molecule for energy storage in living organisms is ATP. With its unique structure containing high-energy phosphate bonds, ATP is able to efficiently store and transport chemical energy within cells. Through cellular respiration, photons from the sun are transformed into chemical energy and stored in the bonds of ATP. This stored energy can then be quickly tapped and transferred to power a multitude of essential biological processes. Without the energy storage capacity of ATP, life as we know it simply could not exist.
ATP powers everything from muscle contraction and nerve impulses to the synthesis of proteins and nucleic acids. This molecule is continuously recycled within cells, providing a mobile energy currency that can rapidly deliver power wherever and whenever it is needed. The central role of ATP highlights how life has evolved sophisticated methods to harness energy from the environment and regulate its usage. Energy storage in ATP remains one of the most fundamental requirements of all biological systems.