What Is Radiated Heat?
What is Radiated Heat?
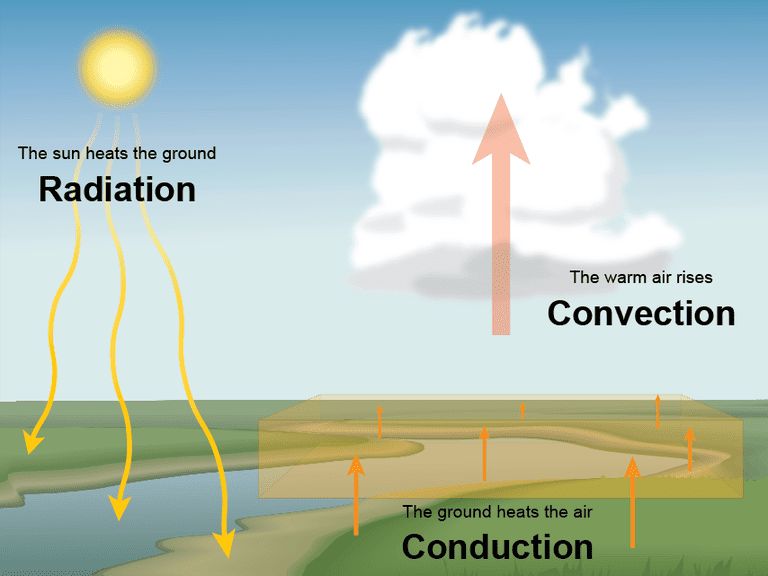
Radiated heat, also known as thermal radiation, refers to electromagnetic waves emitted by objects due to their temperature. All objects with a temperature above absolute zero emit thermal radiation. This radiation does not require a medium to travel through and can move directly from the hot object to something colder through empty space.
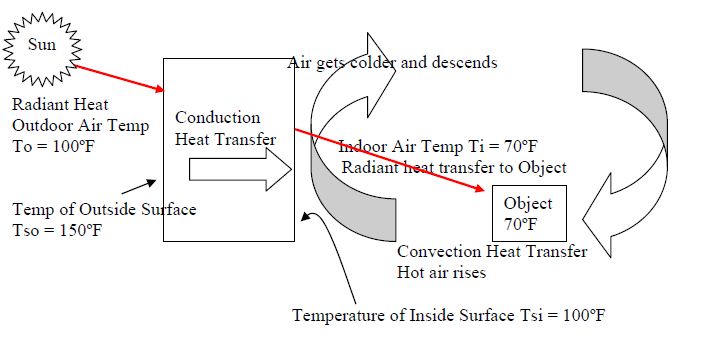
Radiated heat transfer occurs when infrared radiation is emitted by one body and absorbed by another. The hotter the body, the shorter wavelength and higher frequency the radiation will have. When this radiation hits the cooler body, it is absorbed and heats it up. The radiation works to equalize the temperature between the two objects.
Radiated heat is different from conduction and convection heat transfer. Conduction is the transfer of heat between objects in direct contact with each other, like a pot on a stove. Convection is the transfer of heat through the movement of fluids or gases, like hot air rising or boiling water. Radiated heat does not rely on direct contact or fluid movement to transfer thermal energy.
Examples of Radiated Heat
Radiated heat is all around us in our everyday lives. Here are some common examples:
The sun warming the earth – The sun produces intense heat and light through nuclear fusion reactions. This energy is transmitted across empty space to the Earth in the form of radiated heat and light. The infrared radiation from the sun warms the land, oceans, and atmosphere – making life on Earth possible.
Infrared heaters – Infrared heaters emit infrared radiation that is absorbed by objects, causing them to warm up. Unlike convection heaters that heat the air, infrared heaters directly heat the objects they shine on through radiation.
Hot stovetops/ovens – The electric coils or gas burners of stovetops and the heating elements of ovens generate high heat that gets transferred to pots, pans, and food mainly through infrared radiation. This allows for efficient and fast cooking.
How Radiated Heat Travels
Radiated heat travels in straight lines from hot objects to cooler objects. Unlike conduction or convection, radiated heat does not require any medium like air or water to transfer the heat. The heat is transported by electromagnetic waves that can propagate through empty space.
When the electromagnetic waves encounter an object, the waves can be absorbed, transmitted through, or reflected off the object, depending on the material. An ideal “black body” absorbs all the radiated heat that reaches it, while a reflective surface like a mirror will reflect the majority of the radiated heat.
In summary, radiated heat travels directly from hot to cold objects without relying on any intermediate matter. The waves can be absorbed, transmitted, or reflected according to the composition of the object they encounter.
Radiated Heat Transfer Rate
The rate at which radiated heat is transferred depends on two key factors:
Temperature difference between objects – The greater the temperature difference between two objects, the faster heat will be radiated from the warmer object to the cooler object. This is because the radiated heat flux increases with the difference in absolute temperatures between the objects to the power of four, according to the Stefan-Boltzmann law.
Material emissivity – The emissivity of a material determines how readily it emits radiated heat. Emissivity ranges from 0 to 1, with 1 being a perfect blackbody that emits the maximum amount of radiation. Materials with higher emissivity will transfer radiated heat faster than materials with lower emissivity.
Therefore, the rate of radiated heat transfer increases significantly with larger temperature differences and when the materials involved have high emissivity levels. Thermal engineers take both factors into account when designing systems to control radiated heat transfer.
Applications of Radiated Heat
Radiated heat has many practical applications in our everyday lives. Some of the most common uses are:
Heating of buildings and food: Radiated heat is commonly used for indoor heating. Furnaces, boilers, and other heating systems rely on radiated heat to warm buildings. Radiators and baseboard heaters transfer heat through radiation. Similarly, radiated heat from ovens and grills cooks food.
Night vision technologies: Devices like night vision goggles detect radiated infrared light to enhance vision in low-light conditions. They convert this infrared radiation into visible images.
Remote temperature sensing: Infrared thermometers measure surface temperatures from a distance. They detect the infrared radiation emitted by objects to determine their temperature without contact. This allows temperature measurement of hazardous or hard-to-reach objects.
Radiated Heat vs Convection
Radiated heat and convective heat transfer are two distinct mechanisms of thermal energy transfer. The key differences between them are:
– Convection relies on the bulk motion of a fluid (air or liquid) to transfer heat, whereas radiation does not require a medium to travel through.
– Convective heat transfer decreases logarithmically with distance from the heat source, whereas radiative heat transfer follows the inverse square law, decreasing rapidly with distance.
– Convection involves the macroscopic movement of matter, while radiation works at the microscopic, electromagnetic wave level.
– Convection can be natural (driven by buoyancy forces) or forced (driven by external means like a fan). Radiation does not rely on forces or motion.
– Convective heat flows horizontally and vertically, while radiative heat travels in straight lines radially outward from the source.
Understanding these key differences allows engineers to account for both radiation and convection when designing systems like heating, ventilation, and air conditioning.
Radiated Heat vs Conduction
There are a few critical differnences between how radiated heat and conductive heat work. Conduction relies on molecular contact between objects to transfer heat energy, whereas radiation can transfer heat across large distances, even across vacuums, without direct contact between objects.
For conduction to occur, the objects exchanging heat energy must be touching in some way to allow molecular transmission of kinetic energy. The molecules of the hotter object collide with the cooler object, energizing the molecules of the cooler object through direct contact. Radiated heat, on the other hand, can travel through empty space as electromagnetic waves without relying on molecular contact. This allows radiated heat to transfer energy over long distances.
Additionally, conduction requires matter to work. If there is no matter present, such as in a vacuum, conduction cannot occur since there are no molecules available to collide and pass on kinetic energy. Radiated heat, however, can travel through vacuums since it does not rely on matter to propagate. The ability to cross vacuums allows radiated heat to even transfer between objects in space.
In summary, radiated heat relies on electromagnetic radiation, while conducted heat relies on molecular collisions. This key difference allows radiated heat to transfer energy over large distances without contact between objects, even through complete vacuums. Conduction, on the other hand, is limited by the need for physical matter to touch for heat to propagate.
Controlling Radiated Heat
There are several ways to control or reduce the flow of radiated heat in various applications:
Insulation Materials – Special insulation materials like fiberglass, foam, and wool can slow the transfer of radiated heat. The air pockets within these materials reflect the infrared radiation. Installing insulation in walls, ceilings, and attics reduces heating and cooling costs in homes and buildings.
Reflective Surfaces – Radiated heat can be reflected using shiny, polished metal surfaces like aluminum foil. The smoother and more polished the material is, the better it will reflect radiated heat. Reflective insulation is used in building construction and even spacecraft.
Glass – Glass is transparent to sunlight but opaque to longwave infrared radiation from objects. This makes it effective for trapping radiated heat, such as in greenhouses. The glass acts like insulation, keeping the interior warm while the outside remains cold.
Safety and Radiated Heat
When it comes to radiated heat, safety should always be a top concern. Radiated heat can pose risks like heat illness/burns, infrared radiation dangers, and fire hazards if proper precautions aren’t taken.
As we absorb radiated heat from sources like the sun or hot machinery, it’s easy to become overexposed. This can lead to heat cramps, heat exhaustion, or heat stroke in serious cases. Wearing protective clothing, staying hydrated, and limiting exposure are key to preventing heat related illness. Burns are also a risk if skin makes direct contact with hot radiating sources.
Infrared radiation from radiated heat can damage skin and eyes if exposure is excessive. IR radiation is classified as a carcinogen by organizations like the WHO. Limiting contact with strong IR sources and wearing protective eyewear are important safety steps.
The high temperatures that produce radiated heat also present fire hazards. Ensure radiating heat sources like heaters or industrial equipment are kept away from flammable materials. Additionally, make sure electrical devices that emit radiated heat do not overload circuits or wiring that could spark fires.
With proper usage and safety precautions, the risks of radiated heat can be minimized. But it’s always crucial to be mindful of the dangers excessive radiated heat can pose to human health and fire safety.
Fun Facts About Radiated Heat
Radiated heat transfer was first discovered in the early 19th century by British polymath John Leslie. Leslie conducted experiments showing that heat could be transferred through empty space, disproving previous theories that required a medium like air or water. This discovery paved the way for our modern understanding of thermal radiation.
The highest radiated temperature artificially achieved on Earth is around 3.6 billion Kelvin! This was accomplished at the Large Hadron Collider in Switzerland. When lead ions were smashed together, their kinetic energy was converted into thermal radiation emitting a temperature orders of magnitude hotter than the core of the sun.
Radiated heat plays a crucial role in maintaining Earth’s energy balance. The sun radiates heat and light to Earth constantly. Our planet absorbs some of this energy and radiates a portion back out into space. This exchange of incoming and outgoing radiation regulates Earth’s climate and allows life to thrive.