What The Thermal Energy Of An Object Depends On?
Thermal energy is the total kinetic and potential energy of the atoms and molecules that make up an object. This energy causes objects to feel hot or cold and determines how easily heat can be transferred between objects.
The thermal energy of an object depends on several factors, including temperature, mass, specific heat capacity, phase changes, chemical composition, surface area, surroundings, and motion. This article will provide an overview of each of these factors and explain how they impact the thermal energy of an object.
1. Temperature
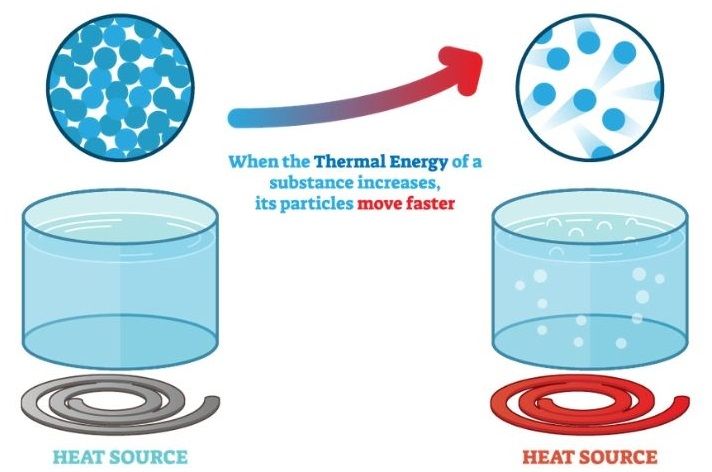
The main factor that determines an object’s thermal energy is its temperature. An object’s temperature is a measure of the average kinetic energy of its particles. As an object gains thermal energy, its particles vibrate and move faster, increasing the object’s temperature. Similarly, as an object loses thermal energy, its particles slow down and its temperature decreases.
Particles with more kinetic energy correspond to higher temperatures, while particles with less kinetic energy equal lower temperatures. This means that objects with higher temperatures contain more thermal energy in the form of particle motion. For example, boiling water has particles moving very rapidly and has more thermal energy than a block of ice, where the water molecules move much slower.
So in summary, temperature serves as a direct indicator of the thermal energy contained within an object. Higher temperatures mean more total kinetic energy of particles, which equals greater thermal energy. Monitoring an object’s temperature provides insight into its current thermal energy state.
Mass
The mass of an object determines how much thermal energy it can absorb and store. More massive objects have a greater capacity to store thermal energy compared to less massive objects of the same material. This is because thermal energy capacity is directly proportional to mass.
For example, imagine two cubes made of the same metal – one with 1 kg mass and the other with 2 kg mass. If both cubes undergo the same temperature change, say from 20°C to 80°C, the more massive cube will absorb and contain twice as much thermal energy as the less massive cube. This is because the 2 kg cube has twice the mass and therefore twice the capacity to store that energy.
The reason mass affects thermal capacity is because thermal energy is stored in the atoms and molecules that make up an object. More mass means more atoms and molecules available to store thermal energy. So increasing an object’s mass increases the amount of energy it can hold when its temperature changes.
In summary, a more massive object, like a large boulder versus a small rock, will absorb and contain more thermal energy for a given temperature change. Mass is directly related to thermal capacity, so heavier objects can store more heat energy compared to lighter objects of the same material.
Specific Heat Capacity
Specific heat capacity, often abbreviated as specific heat, is the amount of heat energy required to raise the temperature of 1 gram of a substance by 1°C. This property is intrinsic to the molecular structure of a substance.
Substances with a high specific heat require more heat energy to increase in temperature. For example, water has a very high specific heat capacity of 4.18 J/g°C. This means it takes 4.18 joules of heat energy to raise the temperature of 1 gram of water by 1°C. This explains why water is effective at stabilizing temperature. The oceans, which contain enormous volumes of water, require vast amounts of heat energy to change temperature even slightly.
Substances like metals, on the other hand, have lower specific heats. For example, iron’s specific heat capacity is only 0.45 J/g°C. This means iron will increase in temperature much more quickly when heat is applied, compared to water. The specific heat capacity is a major factor determining how fast an object’s thermal energy can change.
Phase Changes
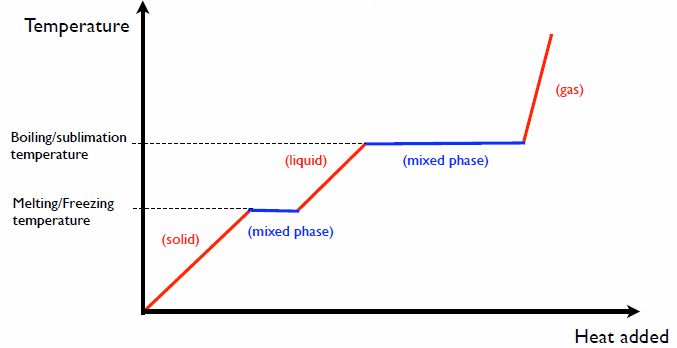
When a substance undergoes a phase change, such as from a solid to a liquid or a liquid to a gas, thermal energy is absorbed or released. This is because energy is required to break the bonds between the molecules in order to change the phase of the substance.
For example, when water is heated and changes from a solid (ice) to a liquid (water), the heat energy is being used to break the crystalline structure of the ice, turning it into liquid water. This process requires a lot of energy, which is absorbed from the surroundings. That’s why heating a pot of ice water feels colder at first – the ice is absorbing thermal energy as it melts.
The reverse process happens when water condenses from a gas (water vapor) to a liquid. The water molecules release energy as they form bonds with each other, turning back into liquid water. This released energy is transferred as thermal energy to the surroundings, causing the surroundings to feel warmer.
The amount of thermal energy required for a phase change depends on the substance. Each substance has a distinct melting point, boiling point, and heat of fusion/vaporization that determines how much thermal energy is absorbed or released when it changes phase. But in all cases, phase changes involve energy transfers that affect the thermal energy of the substance and its surroundings.
Chemical Composition
The chemical composition of a material significantly influences its thermal properties. This is because chemical bonds between atoms determine how much energy is required to raise the temperature of the material.
Materials like metals are composed of atoms bonded together with strong metallic bonds. These bonds can vibrate and absorb thermal energy, but it takes a lot of energy to break them. That’s why metals feel cold – they absorb energy from your skin without increasing much in temperature.
Non-metals like plastics and ceramics are made of molecules with covalent bonds between atoms. These bonds are stronger than metallic bonds, so even more energy is required to raise the temperature. That’s why plastics and ceramics make good insulators.
Materials like water contain molecules with hydrogen bonds. These are weak bonds that can break and reform easily as energy is absorbed, allowing for large changes in temperature with the addition of only a small amount of energy. This explains why water has such a high specific heat capacity.
The strength of chemical bonds between atoms directly correlates with how much energy is required to raise the temperature. Therefore, the chemical composition of a material is a key factor determining its thermal properties.
Surface Area
The surface area of an object plays an important role in thermal energy transfer. As the surface area increases, there is more area for heat to be transferred between the object and its surroundings. This allows the object to absorb or lose thermal energy more readily.
For example, a pot of water with a larger surface area will heat up faster on a stove than the same amount of water in a tall, narrow pot. The larger surface area pot has more area exposed to the heat source so it can absorb thermal energy faster. Similarly, hot coffee in a wide mug will cool down faster than coffee in a tall, insulated travel mug because it has more surface area exposed to the cooler air.
Particles on the surface of the object are freer to gain or lose kinetic energy than particles within the bulk of the material. So increasing surface area increases the number of particles at the surface that can undergo heat transfer. This is why crushed ice melts faster than ice cubes – the crushed ice has more surface area.
In engineering and industrial applications, fins or ridges are added to surfaces to increase the surface area and improve heat dissipation. The high surface area allows heat to be exchanged with the surroundings more efficiently. So the thermal energy of an object depends greatly on its surface area exposure.
Surroundings
An object’s surroundings play a key role in determining its thermal energy. Thermal energy refers to the total kinetic energy of all the molecules within an object. This kinetic energy is directly related to the object’s temperature. The molecules in a hotter object move faster and have more kinetic energy than the molecules in a colder object.
The temperature of an object’s surroundings affects how quickly heat transfers between the object and the environment. Heat flows spontaneously from warmer surroundings to colder objects. For example, when you leave a hot cup of coffee on a table, the coffee transfers heat to the cooler surrounding air, table surface, etc. This heat transfer causes the coffee to cool down over time as thermal energy is lost to the environment.
The rate of heat transfer depends on the temperature difference between the object and its surroundings. The greater the temperature difference, the faster heat transfer occurs. A hot cup of coffee left outside on a cold winter day will lose thermal energy and cool much faster than on a warm spring afternoon because of the larger temperature difference with the cold winter air.
An object’s surroundings don’t necessarily refer just to air temperature. Anything an object is in contact with constitutes its surroundings – like the ground, walls of a room, objects touching it, etc. All these points of contact allow pathways for heat to transfer from areas of higher temperature to lower temperature.
In summary, colder surroundings “drain” thermal energy from hotter objects through heat transfer. The temperature gradient between an object and environment directly affects the object’s thermal energy over time. Consideration of surroundings is key to understanding thermal energy.
Motion
The thermal energy of an object is closely related to the motion of its molecules. As an object gains thermal energy, its molecules vibrate and move faster. This increased molecular motion corresponds to a rise in temperature.
On a microscopic level, temperature is a measure of the average kinetic energy of molecules. Higher temperatures mean the molecules have more kinetic energy and move quicker on average. This motion encompasses vibrations, rotations, and translations of the molecules.
As more thermal energy is added to an object, the molecular motion intensifies. The molecules vibrate more rapidly, rotate faster, and translate across greater distances. This heightened molecular activity spreads the energy throughout the object, raising its overall thermal energy.
In contrast, subtracting thermal energy slows the molecular motion. The molecules have less kinetic energy, so their vibrations, rotations, and translations decrease. This leads to a drop in temperature.
Understanding this link between molecular motion and thermal energy allows scientists to engineer materials at the atomic level to have desired thermal properties. It also explains phenomena like heat diffusion and heat transfer on a deeper level.
Conclusion
In summary, the thermal energy of an object depends primarily on several key factors:
- Temperature – Objects with higher temperatures contain more thermal energy.
- Mass – More massive objects contain more thermal energy.
- Specific heat capacity – Materials with higher specific heat capacities require more energy to change temperature.
- Phase changes – Significant amounts of energy are required to change an object’s state.
- Chemical composition – The types of molecular bonds affect thermal energy.
- Surface area – More surface area allows faster heat transfer.
- Surroundings – Interactions with the environment impacts thermal energy.
- Motion – Faster motion corresponds to greater kinetic energy.
By understanding how these different factors influence the thermal energy of an object, we can better comprehend thermodynamic principles and work with thermal systems. The key takeaway is that many variables are at play in determining the heat content of matter.