Is Heat Same As Thermal Energy?
Heat and thermal energy are two concepts that are often used interchangeably in everyday language. However, in thermodynamics, they refer to distinct scientific definitions. Understanding the precise difference between heat and thermal energy is important for interpreting the laws of thermodynamics correctly.
Thermal energy refers to the total internal kinetic and potential energy of all the molecules within an object. The faster the molecules vibrate and move, the more thermal energy they possess. Heat is energy transferred between objects or systems due to a temperature difference. Heat flows spontaneously from a hotter to a colder body. While related, heat and thermal energy are not the same thing.
Definitions
Heat and thermal energy are related concepts, but have distinct meanings in physics and thermodynamics.
Heat refers to the transfer of thermal energy between substances due to a temperature difference. It flows spontaneously from a hotter substance to a colder substance. Heat is not a property of a system, it is the flow of thermal energy driven by entropy.
Thermal energy is the total internal kinetic and potential energy of all the molecules within an object. It relates to the total random motion of particles and vibrations in a substance. Thermal energy is a state function and depends on factors like temperature, mass, and chemical composition. It is an extensive property.
Relationship Between Heat and Thermal Energy
Heat and thermal energy are closely related, but they are not exactly the same thing. Heat refers specifically to the transfer of thermal energy between objects or systems. Thermal energy, on the other hand, refers to the total kinetic energy of molecules within an object or system.
Thermal energy exists on a microscopic scale – it is the energy associated with the random motions of atoms and molecules. The higher the temperature of a substance, the greater the thermal energy since the atoms and molecules are moving faster. Heat occurs when there is a difference in temperature between two objects or systems that allows thermal energy to flow from the higher temperature to the lower temperature object.
For example, when you place a hot pot on a stove burner, thermal energy flows as heat from the burner into the pot, increasing the thermal energy and temperature of the pot. The greater the temperature difference between the burner and the pot, the faster the heat transfer occurs. Thermal energy is always conserved, but heat will continue transferring until thermal equilibrium is reached.
So in summary – thermal energy is the total energy of molecular motion within a substance while heat is the transfer of thermal energy between substances. You cannot directly measure thermal energy, but you can calculate and measure heat flow and temperature change. Understanding this key distinction is important for the study of thermodynamics.
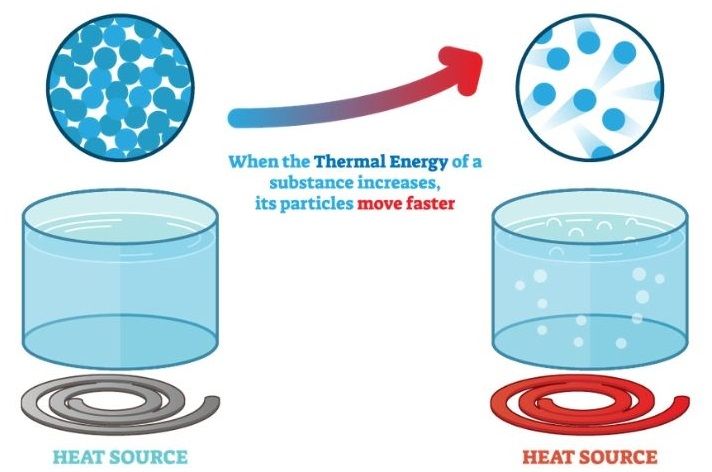
Measuring Heat vs Thermal Energy
Heat and thermal energy are closely related, but there are some key differences in how they are measured scientifically:
Heat is measured in units of joules or calories. Joules are the standard SI unit for measuring heat, while calories are the older non-SI units still sometimes used. The amount of heat is measured by how much energy is transferred from one system or object to another due to temperature differences between them.
Thermal energy, on the other hand, is measured in joules only. Thermal energy refers to the total kinetic energy of all the molecules and atoms in an object. This depends on the temperature, mass, and material composition. So while related, heat specifically measures energy transfer, while thermal energy measures total molecular kinetic energy.
Understanding the distinction between how heat and thermal energy are quantified can shed light on their relationship. While closely linked, the exact units and methods used to measure heat versus thermal energy elucidate the nuanced differences between the two thermodynamic concepts.
Examples
To understand the relationship between heat and thermal energy, it’s helpful to look at some everyday examples:
Boiling water example:
When you boil water on a stove, you are adding heat to the water by heating it up. This added heat causes the water molecules to move faster and spread farther apart. The higher kinetic energy of the faster moving molecules is thermal energy. So by adding heat to the water, you are increasing its thermal energy and causing it to boil.
Ice melting example:
When you melt ice by letting it sit at room temperature, heat transfers from the room to the ice, causing the ice molecules to move faster. This added kinetic energy is thermal energy. As more heat is added, the thermal energy continues to increase until the ice melts into liquid water. This example shows that adding heat resulted in an increase in thermal energy in the ice.
Laws of Thermodynamics
The laws of thermodynamics describe the relationships between thermal energy, heat, and other forms of energy. The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed in an isolated system. This means that while energy can change forms, the total amount of energy in a closed system remains constant.
The second law of thermodynamics describes the natural direction of heat transfer and the concept of entropy. It states that heat will spontaneously flow from a hotter object to a colder object. This means that two objects in contact with each other will reach thermal equilibrium over time as heat transfers from the hotter object to the colder object until their temperatures equalize. The second law also shows that it is impossible for heat to flow from a colder object to a hotter object without an external force. This law describes the irreversibility of natural processes.
Heat Transfer
Heat can be transferred in three main ways – conduction, convection, and radiation.
Conduction is the transfer of heat between substances that are in direct contact with each other. Heat flows from the higher temperature substance to the lower temperature one until they reach thermal equilibrium. The rate of conductive heat transfer depends on the temperature difference and the properties of the materials in contact.
Convection is the mode of heat transfer through fluids (liquids and gases). It relies on the circulation and movement of the fluid to transfer heat. As the fluid moves, hotter regions transfer heat to cooler areas. Convection can be natural (caused by temperature differences) or forced (caused by external factors like fans).
Radiation is the transfer of heat via electromagnetic waves, without requiring direct contact between the heat source and destination. All objects emit and absorb radiant energy depending on their temperature. Thermal radiation does not rely on any medium for transfer and can even occur in a vacuum.
Applications
Heat and thermal energy have many important applications in our everyday lives:
Cooking
The heating of food is essential for cooking. Heat is applied to ingredients through various methods like baking, frying, grilling etc. This heat causes chemical and physical changes in the food resulting in cooked and palatable meals.
Engines
Heat and thermal energy are integral to the working of engines. In internal combustion engines like in cars, heat is generated by the burning of fuel. This thermal energy creates pressure that moves the pistons, providing the power to drive the engine.
Heating and Cooling
Heating systems like furnaces, boilers, heaters use fuel combustion or electricity to generate heat which is circulated around living and work spaces. Air conditioners and refrigerators remove heat from the air or substances for cooling purposes.
Weather
The sun is the primary source of heat and thermal energy that impacts weather on Earth. Heat drives convection currents in the atmosphere and oceans creating winds and ocean currents. It also drives the water cycle through evaporation and condensation.
Common Misconceptions
There are a few common misconceptions when it comes to understanding the relationship between heat and thermal energy:
Heat and temperature are not the same
While related, heat and temperature are distinct properties. Temperature measures the average kinetic energy of molecules, indicating how hot or cold an object is. Heat is the transfer of thermal energy from one object or system to another caused by a temperature difference. An object may gain thermal energy without increasing temperature if it undergoes a phase change.
Thermal energy of object doesn’t change with heat transfer
When two objects at different temperatures come in contact, heat transfers from the hotter to the colder object until they reach thermal equilibrium. The thermal energy of the hotter object decreases while the thermal energy of the colder object increases, though the total thermal energy remains constant in accordance with the law of conservation of energy.
Conclusion
In summary, while heat and thermal energy are related concepts, they have distinct meanings in thermodynamics. Heat refers to the transfer of thermal energy between objects or systems due to a temperature difference. Thermal energy is the total internal kinetic and potential energy of all the molecules within an object. Measuring heat vs thermal energy involves different units and calculations.
The key takeaways are:
- Heat is energy in transit while thermal energy is stored internal energy.
- Heat flows spontaneously from objects at higher temperatures to objects at lower temperatures.
- Thermal energy depends on the temperature, mass, and composition of an object.
- Heat capacity and specific heat capacity determine how much thermal energy is required to increase an object’s temperature.
- The laws of thermodynamics govern heat transfer and changes in thermal energy in closed systems.
- Understanding the relationship between heat and thermal energy is essential for many scientific and engineering applications.