How Do You Convert Energy Into Thermal Energy?
What is Thermal Energy?
Thermal energy refers to the internal energy present in matter that arises from the motion and interactions of molecules and atoms. It is directly associated with the temperature of matter. The higher the temperature, the greater the thermal energy. Thermal energy flows from objects at higher temperatures to objects at lower temperatures.
We encounter thermal energy in our everyday lives. For example, the warmth we feel when sitting near a fireplace or touching a warm drink comes from thermal energy transferring from the fire or hot liquid to our bodies. Similarly, the heat that allows a stove to cook food and a car engine to run comes from thermal energy. Other common examples are the heat radiated from hot pavement on a sunny day, the cooling sensation of ice, and the warmth provided by clothing and blankets.
Forms of Energy that Can be Converted to Thermal Energy
There are several common forms of energy that can be converted into thermal energy:
Electrical Energy
Electricity is the flow of electrons, which can generate thermal energy through resistance. As electrons flow through a conductive material like a wire, they collide with atoms, dissipating some energy in the form of heat. Devices like electric stoves, toasters, and space heaters are designed to efficiently convert electrical energy into thermal energy.
Radiant Energy (Light)
Light is a form of radiant energy that can be absorbed by surfaces and converted to thermal energy. For example, sunlight shining on a solar panel gets converted to heat as well as electricity. The thermal energy from sunlight can be used for heating buildings, water, and other applications.
Mechanical Energy (Motion/Friction)
The movement and interaction of objects and materials can produce thermal energy through friction. For instance, rubbing your hands together generates heat through the friction between your palms. Similarly, mechanical brakes convert motion into heat by the friction between brake pads and rotors. Engines also rely on friction to convert the linear motion of pistons into thermal energy.
Chemical Energy
Chemical reactions involve breaking and reforming molecular bonds, which releases or absorbs energy. Exothermic reactions like combustion produce thermal energy, while endothermic reactions require heat input. Burning fuel sources like natural gas, gasoline, and biomass efficiently converts their stored chemical energy into high-temperature thermal energy.
Methods of Converting Other Forms of Energy to Thermal Energy
There are several common methods of converting other forms of energy into thermal energy:
Electric Heating
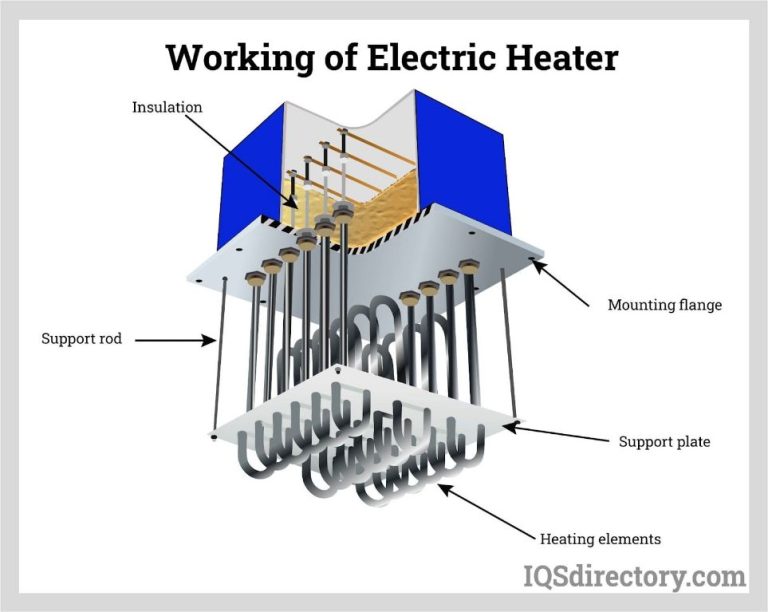
Electric heating converts electrical energy into thermal energy. Devices like electric stoves, space heaters, and water heaters use resistance heating elements that get hot when electricity passes through them. The heat is then transferred to air, water, or whatever else the heating element is in contact with.
Combustion Engines
In combustion engines like those in cars, chemical potential energy stored in fuel is converted into thermal energy through combustion. This thermal energy increases the temperature and pressure of gases, which pushes pistons to provide mechanical work. The majority of the energy released from combustion becomes waste heat.
Friction from Motion
When two surfaces rub against each other, their friction converts mechanical energy into thermal energy. This effect makes brakes on vehicles hot and is a key limitation in many mechanical systems. Insulating parts that rub together and using lubricants can reduce frictional heating.
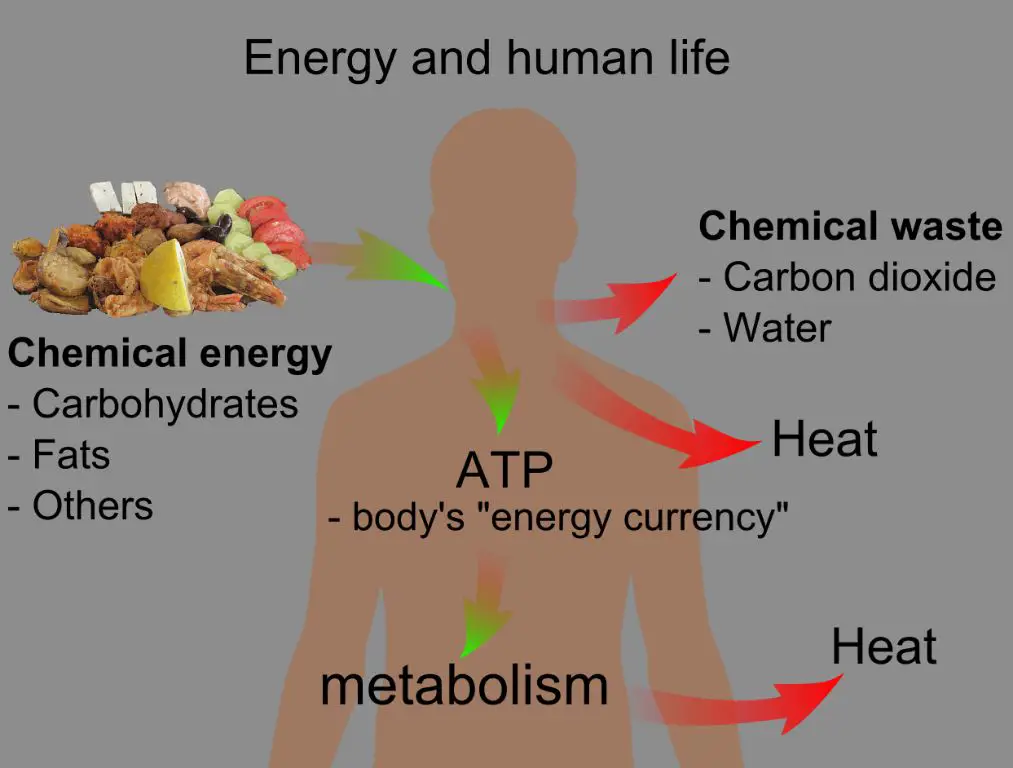
Metabolic Processes in Living Organisms
The chemical reactions within cells that sustain life require energy and generate heat as a byproduct. This allows warm-blooded animals like humans to maintain a consistent internal body temperature. The food energy they consume is chemically converted to thermal energy that keeps their bodies warm.
Using Thermal Energy Efficiently
There are several ways to use thermal energy more efficiently in order to reduce energy waste and costs:
Insulation – Adding insulation to buildings, pipes, boilers and other equipment helps prevent heat loss. Insulation traps air pockets which resist heat flow. Common insulation materials include fiberglass, cellulose, polystyrene and polyurethane foam.
Recovering Waste Heat – Waste heat from industrial processes, power plants, vehicles and appliances can be recovered and reused rather than released into the environment. Heat exchangers, heat pumps and cogeneration systems help capture this waste thermal energy.
Heat Pumps – Heat pumps move thermal energy from one place to another instead of directly generating it. For example, air-source heat pumps can extract heat from outdoor air and pump it indoors for heating. They can also work in reverse to cool indoor air.
Using methods like insulation, waste heat recovery and heat pumps allows thermal energy to be used more efficiently and reduces the amount of extra energy that needs to be generated for heating and cooling.
Examples of Devices that Convert Energy to Thermal Energy
Many common household and industrial devices convert other forms of energy into thermal energy as part of their normal operation. Here are some notable examples:
Space Heaters
Space heaters are designed to convert electrical energy into thermal energy to heat the surrounding air. They contain resistive heating elements that get hot when electricity passes through them. This heat is dissipated into the room.
Internal Combustion Engines
The operation of internal combustion engines, like those in cars, relies on the conversion of chemical energy in fuel into thermal energy through combustion. This thermal energy creates pressure that moves the pistons, providing mechanical energy.
Electric Stoves/Ovens
Electric stoves and ovens use resistive heating coils to convert electrical energy into thermal energy that is used for cooking and baking. The coils get hot when electricity passes through them.
Light Bulbs
Incandescent and halogen light bulbs work by converting electrical current into thermal energy inside the filament, which gets hot and glows to produce light. Only about 10% of the energy is converted into visible light.
Human Bodies
The human body is constantly converting chemical energy from food into thermal energy to maintain body temperature. Excess thermal energy is dissipated into the environment through convection, sweating, and radiation.
Measuring Thermal Energy
Thermal energy can be measured in a few common units:
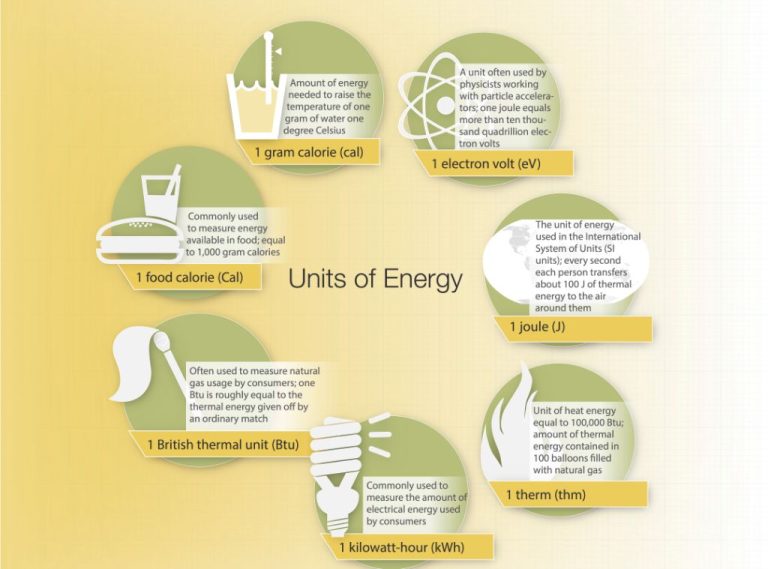
- Calories (cal) – The amount of energy needed to raise 1 gram of water 1 degree Celsius.
- British Thermal Units (BTU) – The amount of energy needed to raise 1 pound of water 1 degree Fahrenheit. 1 BTU is about 1,055 joules.
- Joules – The SI unit of energy. 1 calorie is equal to 4.184 joules.
These units allow us to quantify thermal energy for calculations and comparisons. We can measure thermal energy in practice using thermometers and thermostats.
Thermometers use the expansion and contraction of materials in response to temperature changes to measure the thermal energy or temperature of a system. Common types include mercury, alcohol, and digital thermometers. They can measure temperatures on the Celsius, Fahrenheit, or Kelvin scale.
Thermostats contain thermometers and control systems to regulate thermal energy in heating and cooling systems. They sense the current temperature, compare it to the desired temperature set by users, and signal heating or cooling devices to switch on or off as needed to reach the setpoint.
Accurate temperature measurements from thermometers and thermostats allow us to monitor and control thermal energy effectively in various applications.
Thermal Energy Storage
Thermal energy storage (TES) allows excess thermal energy to be captured and stored for later use. There are two main types of thermal energy storage: sensible heat storage and latent heat storage.
Sensible Heat Storage
Sensible heat storage involves raising or lowering the temperature of a storage medium. Examples of storage mediums include water, rocks, sand, molten salts, concrete and earth. The amount of thermal energy stored depends on the specific heat capacity of the medium, its mass and the temperature change. Sensible heat storage is used in solar water heaters and passive solar heating systems.
Latent Heat Storage
Latent heat storage takes advantage of the energy absorbed or released when a storage medium undergoes a phase change, such as from solid to liquid. Latent heat storage materials include paraffin waxes, fatty acids and salt hydrates. These materials can store large amounts of thermal energy per unit mass. Latent heat storage is often used in solar heating applications.
Applications of Thermal Storage
Some common applications of thermal energy storage include:
- Storing solar thermal energy from solar collectors for use at night in space heating or domestic hot water systems
- Storing excess thermal energy from power plants or industrial processes for later electricity generation or process heating
- Storing winter cold in underground pits or boreholes for summer air conditioning
Thermal storage allows energy to be captured when available and used when needed, increasing efficiency and reducing energy waste.
Thermal Energy Transfer
Thermal energy can be transferred between objects or locations through three main mechanisms: conduction, convection, and radiation.
Conduction
Conduction is the transfer of thermal energy between objects that are in direct physical contact with each other. It occurs when atoms and molecules with higher kinetic energy collide with and transfer some of their energy to neighboring atoms and molecules with lower energy. Metals are good conductors of thermal energy.
Convection
Convection is the transfer of thermal energy by the movement of heated fluid. It occurs in liquids and gases. As the fluid is heated, it expands, becomes less dense, and rises. Cooler, denser fluid then sinks to take its place, creating currents that transfer heat. Examples include hot air rising from a radiator or the circulation of magma in the Earth’s mantle.
Radiation
Thermal radiation is the transfer of thermal energy through electromagnetic waves. All objects emit electromagnetic radiation depending on their temperature. Hotter objects emit more high-energy radiation. Thermal radiation does not require direct contact between objects and can travel long distances through vacuums. The heat we feel from the sun is an example of thermal radiation.
In the real world, these three mechanisms often work together to transfer thermal energy. For example, in a pot of boiling water, conduction transfers heat from the stovetop into the pot, convection currents distribute the heat within the water, and the steam radiates thermal energy into the air.
Thermal Energy and Heat Engines
A heat engine is a device that converts thermal energy into mechanical work. Heat engines operate by exploiting temperature differences. They take in heat at high temperature from a hot reservoir and reject heat at a lower temperature to a cold reservoir, converting part of that heat into useful work in the process.
The efficiency of a heat engine depends on the difference between the hot and cold reservoir temperatures. The greater the temperature difference, the more efficient the engine. The maximum possible efficiency of a heat engine is given by the Carnot efficiency. No real heat engine can operate at this maximum efficiency due to irreversibilities inherent in real engines.
There are several common types of heat engines:
- Internal combustion engines – These use the exothermic combustion of fuel to create high temperature/pressure gases which are permitted to expand, moving a piston to produce work. Examples include gasoline, diesel and gas turbine engines used in vehicles and power plants.
- External combustion engines – These use an external heat source to heat a separate working fluid which then expands to drive a piston or turbine. Examples include steam engines and Stirling engines.
- Heat pumps – These use work to move heat from a colder to a hotter reservoir, effectively “pumping” heat. The most common example is the vapor-compression refrigerator.
Heat engines have enabled the widespread use of vehicles, electricity generation, refrigeration, air conditioning and many other technologies by extracting useful work from thermal energy.
Applications and Impact of Thermal Energy Conversion
Thermal energy conversion has widespread applications that impact many aspects of everyday life. Some of the key uses and impacts include:
Heating and Cooling Homes and Buildings
Thermal energy is essential for heating and cooling residential and commercial buildings. Furnaces, boilers, heat pumps, air conditioners, and other HVAC (heating, ventilation and air conditioning) systems rely on the conversion of energy into thermal energy to maintain comfortable temperatures inside.
Power Generation
Many power plants convert chemical energy in fuels like natural gas, coal, oil, or biomass into thermal energy that is then used to boil water into steam. The high-pressure steam spins turbines connected to generators to produce electricity.
Industrial Processes
Factories use thermal energy conversion for industrial processes like smelting metals, refining petroleum, drying materials, and more. This allows raw materials to be transformed into finished products.
Cooking
Modern stoves, ovens, and other cooking appliances convert the chemical energy in natural gas or electricity into the thermal energy required to heat and cook food.
Transportation
Internal combustion engines in cars, trucks, airplanes and other vehicles burn gasoline, diesel, or other fuels. This chemical to thermal energy conversion propels the vehicle.
In summary, thermal energy conversion enables heating and cooling, power generation, manufacturing, cooking, transportation, and many other essential applications in society today.