What Is Known As Thermal?
What is Thermal Energy?
Thermal energy refers to the internal energy contained within a system due to the motion of its atoms and molecules. It arises from the kinetic energy of atoms and molecules, which is determined by their velocity and vibrational states. All matter contains some amount of thermal energy, as atomic and molecular motion is an intrinsic property of matter. The faster the average speed of atoms/molecules in a substance, the higher its thermal energy.
Thermal energy is often referred to as heat, although heat more specifically refers to the transfer of thermal energy between systems, such as from a hot object to a colder one. Thermal energy itself resides within an object or system and is not in transit. Thermal energy is an extensive property, meaning the total amount depends on the size and composition of the system. It can be quantitatively described by thermodynamic temperature.
Sources of Thermal Energy
Thermal energy comes from various sources, both natural and human-made. Some of the main sources of thermal energy include:
Combustion of fuel
The burning of fuels like natural gas, oil, coal, wood, and biofuels releases a large amount of thermal energy that can be captured and used. This exothermic chemical reaction between a fuel source and oxygen is one of the most common ways humans produce thermal energy.
Friction
Kinetic energy and friction converting into thermal energy is a key source of heat in certain mechanical processes and applications. Friction from brakes and bearings, as well as viscous friction in fluids, results in thermal energy generation.
Electrical energy and resistance heating
Passing an electrical current through a resistor results in Joule heating as energy is lost to thermal energy. Electrical resistance heaters and electrification more broadly enable thermal energy to be produced on demand.
Nuclear/radioactive decay
Radioactive decay of unstable isotopes and nuclear fission/fusion reactions release tremendous amounts of thermal energy that can be harnessed, as is done in nuclear power plants.
Body heat from living organisms
The chemical reactions involved in metabolism generate heat within organisms. Human body heat can be a source of thermal energy for localized applications.
Measuring Thermal Energy
There are a few key ways to measure thermal energy:
Temperature
Temperature is a measure of the average kinetic energy of particles in a substance. It is measured using thermometers and temperature scales like Fahrenheit, Celsius, and Kelvin. Higher temperatures mean particles are moving faster on average.
Specific Heat Capacity
Specific heat capacity describes how much energy is needed to raise the temperature of 1 gram of a substance by 1 degree Celsius. Substances with higher specific heat capacities require more thermal energy to change their temperature.
Calorimetry
Calorimetry is used to measure the amount of heat absorbed or released during a chemical or physical process. This involves precisely measuring the temperature change in substances undergoing heating or cooling. The temperature change is used to calculate the thermal energy based on the specific heat capacity of the substances.
Transferring Thermal Energy
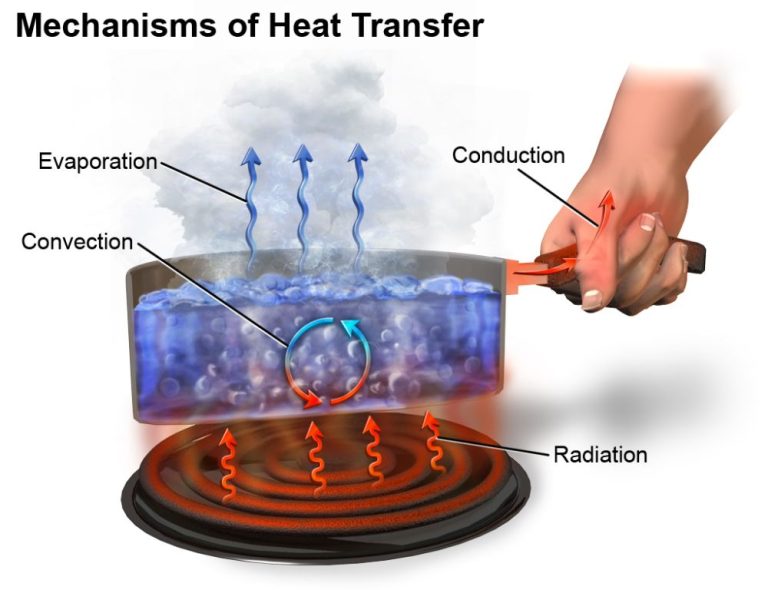
Thermal energy can be transferred between objects or regions through three main mechanisms: conduction, convection, and radiation.
Conduction
Conduction is the transfer of thermal energy between objects or regions that are in direct physical contact with each other. It occurs when faster moving molecules collided with slower moving molecules, transferring kinetic energy. Metals are good thermal conductors.
Convection
Convection is the transfer of thermal energy by the motion of fluids. It occurs in liquids and gases. As the fluid is heated, it expands, becomes less dense, and rises. Cooler, denser fluid then moves to take its place, creating circulation. Convection currents distribute heat through the medium.
Radiation
Radiation is the transfer of thermal energy by electromagnetic waves. All objects emit thermal radiation related to their temperature. Radiation does not require direct contact between objects and can travel through empty space. Radiant energy is absorbed bysurfaces it encounters.
Thermal Energy Storage
Thermal energy can be stored for later use through several methods. The main types are sensible heat storage, latent heat storage, and thermochemical storage.
Sensible heat storage involves raising or lowering the temperature of a storage medium. Examples include heating up water in a tank or bricks in a storage heater. The amount of energy stored depends on the specific heat capacity of the medium, the temperature change, and the amount of storage material.
Latent heat storage relies on materials changing phase from solid to liquid or liquid to gas. As heat is added or removed, the material absorbs or releases large amounts of energy at an almost constant temperature. Molten salts and paraffin waxes are common phase change materials used for latent heat storage.
Thermochemical storage uses reversible chemical reactions that absorb or release heat. For example, metal hydroxides can dehydrate endothermically by losing water and rehydrate exothermically by absorbing water and heat. Thermochemical storage has a higher energy storage density than sensible or latent heat methods.
Thermal Energy Applications
Thermal energy has many important applications in daily life. Some of the most significant uses involve heating and cooling systems, internal combustion engines, electric power generation, industrial processes, cooking, and thermal comfort.
Heating and cooling systems rely on the transfer of thermal energy to regulate indoor temperatures. Furnaces, boilers, heat pumps, air conditioners all work by heating or cooling air or water which is circulated to maintain comfortable temperatures inside homes, offices, and other buildings.
Internal combustion engines found in most vehicles convert thermal energy from burning fuel into mechanical energy to propel the vehicle. The heat causes gases to expand and move pistons to generate power.
In electric power plants, heat is used to boil water to run steam turbines that spin generators to produce electricity. Fossil fuels, nuclear fission, and geothermal heat can all provide the thermal energy to run these turbines.
Many industrial processes involve the application of thermal energy as well. Smelting metals, refining petroleum, drying materials, and chemical synthesis all rely on careful control of heat to drive reactions and transformations.
Cooking is fundamentally an application of thermal energy to transform ingredients into food. The heat causes physical and chemical changes that make food safe and digestible for human consumption.
Finally, thermal comfort refers to maintaining ideal temperatures, humidity, and air flow for human occupancy. Proper thermal conditions keep people healthy and allow them to focus on work and activities without being distracted by feeling too hot or cold.
Thermal Energy and Heat
Thermal energy and heat are closely related concepts in thermodynamics. Thermal energy refers to the total kinetic energy of molecules within a substance, which relates to the vibrational motion of the molecules. The higher the temperature of a substance, the greater the thermal energy because the molecules vibrate faster and have more kinetic energy. Heat is energy transferred between substances due to a temperature difference. It always flows spontaneously from a hotter to a colder body. This occurs because higher kinetic energy molecules collide with lower kinetic energy molecules, increasing their kinetic energy. Thus, heat is the transfer of thermal energy between substances, while thermal energy is contained within a substance.
The relationship is that heat flow increases the thermal energy of the colder substance and decreases the thermal energy of the hotter substance. Thermal energy is an extensive property, meaning the total amount depends on the size and mass of the system. Heat is an intensive property, meaning it depends on temperature difference, not mass. Heat will flow between two objects until they reach thermal equilibrium, meaning their temperatures equalize as the thermal energy evens out. Understanding this relationship is key to thermodynamics and designing efficient heating and cooling systems.
Thermal Energy Efficiency
There are several ways to improve thermal energy efficiency in buildings and industrial processes. Some key methods include:
Insulation
Insulation prevents heat transfer and helps maintain desired temperatures. Common insulation materials include fiberglass, cellulose, polystyrene, and polyurethane foam. Proper insulation in walls, attics, basements, and around pipes and HVAC systems can reduce energy consumption for heating and cooling by up to 50%.
Energy Efficient Designs
Buildings and equipment can be designed to maximize thermal efficiency. Passive solar building design takes advantage of sunlight and natural ventilation to reduce reliance on heating and cooling systems. High efficiency HVAC equipment, boilers, furnaces, and heat exchangers can convert fuel into useful thermal energy with less waste. Smart thermostats and zoned temperature control also help optimize thermal efficiency.
Waste Heat Recovery
Industrial processes often produce waste heat that can be captured and reused instead of released into the environment. Heat exchangers can transfer waste heat to provide building heat or preheat boiler water. Waste heat can also drive turbines to generate electricity. Overall, recovering waste heat improves efficiency, reduces costs, and prevents pollution.
Thermal Energy and the Environment
The production and use of thermal energy has major environmental impacts that must be considered and addressed. The biggest issue is that most thermal energy today comes from the burning of fossil fuels like coal, oil and natural gas. When burned, these fuels release greenhouse gases like carbon dioxide that trap heat and contribute to global warming and climate change.
As the dangers of climate change become more apparent, there is a growing need for renewable sources of thermal energy that do not produce greenhouse gases. Options include solar thermal, geothermal, and waste heat recovery systems that capture heat that would otherwise be lost to the environment.
Even with renewable sources, steps should still be taken to use thermal energy efficiently and reduce waste heat wherever possible. This includes proper insulation in buildings and industrial processes, as well as technology like heat pumps and combined heat and power systems that recycle waste heat. Pursuing energy efficiency is one of the fastest ways to lower greenhouse gas emissions from thermal energy use.
With growing global energy demand, thermal energy will continue to play a major role in society. But it must be transitioned to cleaner sources and used more responsibly to avoid further environmental harm. There are many promising thermal technologies available today, if deployed widely, that can supply humanity’s thermal energy needs in a sustainable manner.
Future of Thermal Science
Thermal science is an exciting and rapidly evolving field. Here are some key areas of advancement to look out for in the future:
Advances in Thermoelectrics
Thermoelectric materials that can convert heat directly into electricity (and vice versa) are becoming increasingly efficient and cost-effective. Novel thermoelectric materials like skutterudites, clathrates, and Zintl phases could boost the performance and scalability of thermoelectric power generation and cooling. Engineered nanostructures and thin films also show promise for high-efficiency thermoelectric energy conversion.
Novel Thermal Materials
New thermal management materials like aerogels, phase change materials, and heat pipes are enabling better control and utilization of thermal energy. For example, ultra-low thermal conductivity aerogels can provide superior insulation, while high thermal conductivity graphites and carbon nanotubes can enhance heat dissipation in electronics. Materials with tunable thermal properties may also allow dynamic control of heat transfer.
Applications in Emerging Technologies
Thermal science will play a key role in technologies like flexible electronics, solid state lighting, and high-power electronics. Advanced thermal interface materials and microscale cooling systems will be needed to manage heat in these devices. Waste heat recovery using thermoelectric generators or absorption chillers could also boost the energy efficiency of everything from data centers to electric vehicles. Overall, thermal engineering will help drive progress in renewable energy, computing, transportation, and other critical emerging technologies.