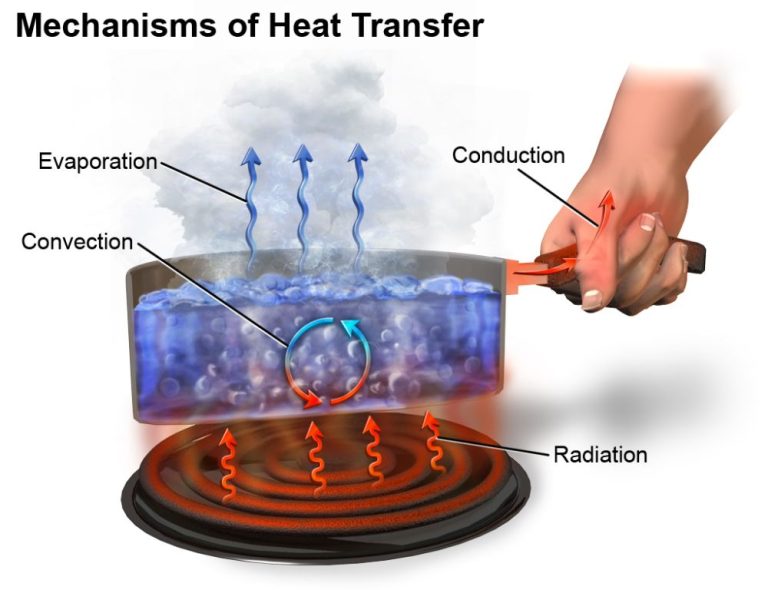
What Are Some Forms Of Heat?
Heat is a form of energy that is transferred from one body or system to another as a result of the temperature difference between them. There are various different forms in which heat energy can exist.
This article explores the main forms of heat energy and provides examples of where they are found in nature and everyday life. The focus will be on thermal/kinetic energy, friction, electric current, chemical reactions, nuclear reactions, electromagnetic radiation, geothermal energy, and metabolism as examples of heat energy.
Thermal/Kinetic Energy
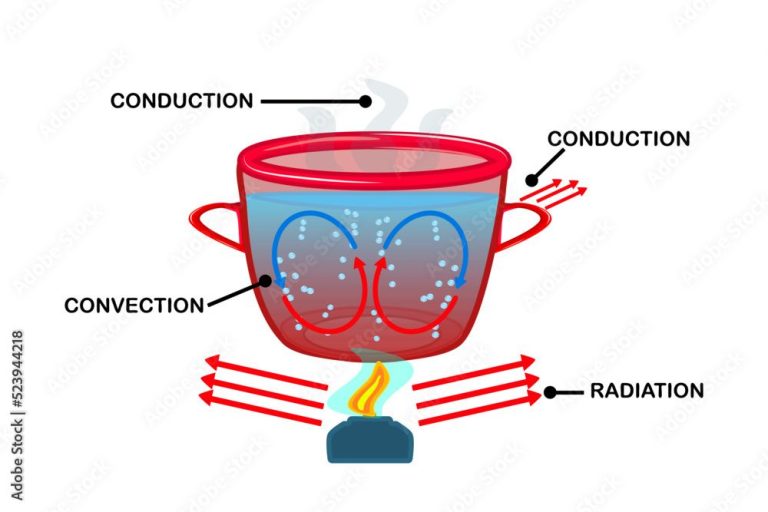
Thermal energy, also known as heat, is the energy transferred between systems or objects due to their temperature difference. On a microscopic scale, thermal energy is the kinetic energy of atoms and molecules in matter. The higher the temperature of matter, the faster the atoms and molecules vibrate and move. Through direct contact between objects, faster moving atoms and molecules transfer some of their energy to atoms and molecules with lower kinetic energy. This transfer of kinetic energy is what we perceive as heat flowing from a hotter object to a colder object.
We encounter thermal energy in our everyday lives. For example, when we boil water on a stove, the thermal energy from the burner flame is transferred to the pot and the water molecules inside it. This added energy makes the water molecules move faster and collide more frequently, heating up the water. Another example is the heat we feel coming off a radiator or heater. The thermal energy is transferred from the heating element to the surrounding air molecules, causing them to speed up and spread the heat around the room.
Thermal energy is one of the most common forms of heat we experience. It arises from the constant random motion of microscopic particles that make up all matter. This atomic and molecular motion is directly linked to temperature, allowing thermal energy to flow spontaneously from higher to lower temperatures.
Friction
Rubbing two objects against each other creates friction between their surfaces. The friction generates heat as the kinetic energy from the motion and pressure between the objects gets converted into thermal energy. The faster the motion and higher the pressure, the more heat that gets produced.
This rise in temperature from friction is something we experience regularly in everyday life. For example, rubbing your hands together warms them up on a cold day. The friction from rubbing produces heat that gets transferred from your hands to your skin. Friction from sports activities and exercise also causes people to get hot and sweaty as motion creates heat.
On a larger scale, mechanical braking systems on vehicles convert motion into heat energy through friction. The brake pads press against the rotating wheels, slowing them down through friction and dissipating the kinetic energy as heat. This allows the vehicle to decrease speed without that energy turning into excessive motion. The heating effect of friction allows brakes to work.
Friction is useful for generating heat in a controlled manner. However, too much friction where it’s not intended can also be problematic, causing damage and inefficiency. Lubricants help reduce friction in mechanical systems when heat is not the goal. Overall, the friction resulting from two surfaces rubbing against each other serves as a basic but highly practical form of heat in our lives.
Electric Current
When an electric current passes through a conductor, it encounters resistance that converts some of the electrical energy into heat.
The flow of electrons through the conductor is impeded by collisions between the electrons and atoms in the material. These collisions cause the atoms to vibrate and heat up, converting electrical energy into thermal energy in the form of heat.
The amount of heat generated depends on the material’s electrical resistance and the amount of current flowing through it. Materials with higher resistance, like tungsten filaments in incandescent light bulbs, can get very hot and glow.
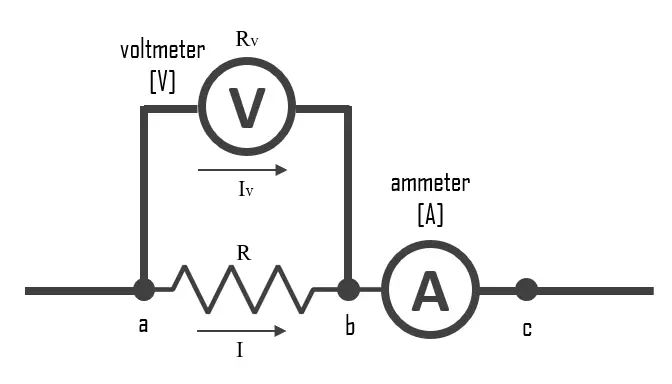
Ohm’s law states that the current flowing through a conductor is directly proportional to the voltage applied and inversely proportional to the resistance. Therefore, higher voltage and lower resistance leads to more current flow, which generates more heat.
Electric heating elements in appliances like toasters, space heaters, and electric stoves rely on this principle to convert electricity into heat for cooking, warming, etc. The resistance in the heating element determines how hot it gets based on the current flowing through it.
Similarly, electrical wiring and components can overheat if too much current passes through. Fuses, circuit breakers, and thermal insulation help prevent fires and damage from excessive electrical resistance heating.
Chemical Reactions
Chemical reactions can produce or absorb heat, depending on whether the reaction is exothermic or endothermic. Exothermic reactions produce heat as a result of the reaction. This occurs when the total energy of the products is less than the total energy of the reactants. The excess energy is released in the form of heat.
Some examples of exothermic chemical reactions that produce heat include:
- Combustion – Burning fuels like wood, coal, oil, and natural gas releases a lot of heat energy.
- Oxidation – Rust formation and cellular respiration are oxidation reactions that give off heat.
- Neutralization – When an acid and base react, it produces water and salt and releases heat.
- Thermite – Iron oxide and aluminum react violently to form aluminum oxide and iron, releasing intense heat.
These exothermic reactions supply heat for many applications, from heating homes and powering engines to cooking food. The energy released can be harnessed in a controlled way for productive uses.
Nuclear Reactions
Nuclear reactions involve changes in the nucleus of atoms that can release enormous amounts of heat energy. There are two main types of nuclear reactions that produce heat:
Nuclear Fission
Nuclear fission is the splitting of a large atomic nucleus into smaller nuclei. This occurs when a neutron strikes the nucleus of a heavy atom like uranium or plutonium, causing it to become unstable and split apart. The fission of uranium atoms has been used to produce electricity in nuclear power plants for decades.
Nuclear Fusion
Nuclear fusion is the joining of two light atomic nuclei to form a heavier nucleus. This reaction requires extremely high temperatures and pressures to overcome electrostatic repulsion between nuclei. Fusion occurs naturally in stars like the sun. Scientists are also working to develop fusion reactors that can create massive amounts of heat and energy on Earth.
Both fission and fusion release energy from the binding forces that hold the nucleus together. The process converts a small amount of mass into a massive amount of heat energy, as described by Einstein’s famous equation E=mc2.
Electromagnetic Radiation
Electromagnetic radiation consists of waves of energy that travel at the speed of light. There are different types of electromagnetic radiation that can transmit heat in the form of infrared radiation, visible light, and ultraviolet rays.
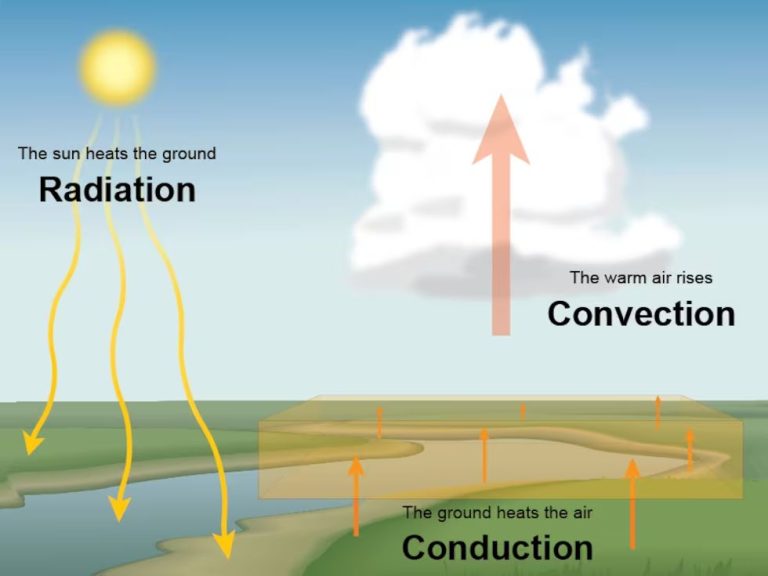
Infrared radiation has longer wavelengths and lower frequencies than visible light. It is invisible to human eyes, but we perceive it as heat. Common sources of infrared radiation include fire, the sun, hot objects, and living organisms. Infrared rays transmit thermal energy that we feel as heat when they are absorbed by our skin.
Visible light from the sun provides heat and illumination. Different colors of light have different wavelengths, frequencies, and energies. Visible light is the most familiar type of electromagnetic radiation that most people encounter in daily life.
Ultraviolet (UV) rays have shorter wavelengths and higher frequencies than visible light. UV rays transmit energy that can have beneficial uses like disinfecting surfaces, but overexposure can be damaging to skin and eyes. The sun is the main natural source of UV radiation.
In summary, infrared radiation, visible light, and UV rays are all forms of electromagnetic radiation that transmit heat and energy at different wavelengths across the electromagnetic spectrum.
Geothermal Energy
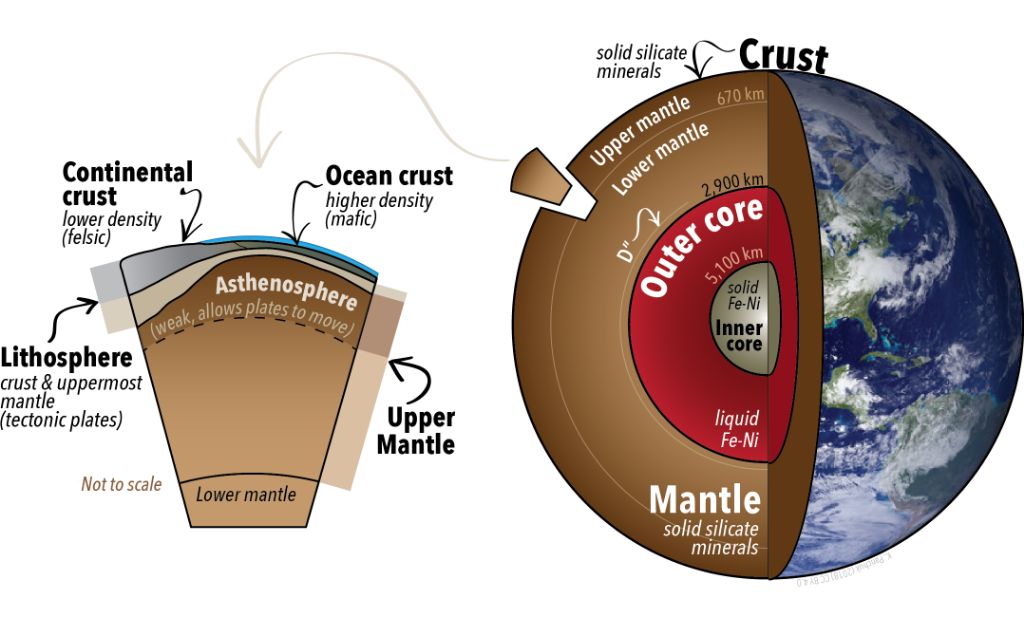
Geothermal energy refers to heat energy generated and stored in the Earth. The geothermal energy present in the Earth’s core, mantle, and crust originates from the original formation of the planet, radioactive decay, and ongoing heat loss. The core of the Earth is the hottest part, with temperatures rising to over 4000°C – hot enough to melt iron. This heat is mainly leftover from planetary accretion during the Earth’s formation, as potential gravitational energy was converted to kinetic heat energy. The heat at the core conducts outwards, leading to high temperatures deep below the Earth’s surface that supply geothermal energy.
Geothermal energy can be harnessed in a few ways. Geothermal power plants can draw hot water or steam from deep underground reservoirs to turn generators and produce electricity. Geothermal heating/cooling systems use geothermal heat pumps to control building temperatures. Direct use applications apply geothermal heat directly, such as heating greenhouses, drying crops, and heating water. Geothermal energy is considered a renewable resource because the Earth continuously produces heat at depth, replenishing the available heat supply.
Metabolism
Metabolism refers to all the chemical reactions that take place within an organism to maintain life. These reactions allow living things to grow, reproduce, repair damage, and respond to their environments. Metabolic processes generate heat as a byproduct of breaking down food and nutrients into energy.
This metabolic heat is essential to maintaining an organism’s body temperature. Endotherms like mammals and birds rely on their metabolism to internally regulate their body heat, while ectotherms like reptiles depend on external heat sources. At the cellular level, metabolic reactions drive processes like muscle contraction that also give off heat.
The rate of metabolism corresponds to the amount of energy being used by the body. Higher metabolic rates generate more heat. Factors like age, body size, and activity levels influence metabolic rate. For example, babies have very high metabolic rates to facilitate rapid growth.
Metabolic heat production varies across organisms, but serves as an important source of thermal energy in all forms of life. This allows living things to maintain homeostasis and carry out the essential functions involving chemical reactions.
Conclusion
In summary, there are several key forms of heat that are important to understand. Kinetic energy or thermal motion of particles is one of the most fundamental sources of heat that drives many other heat-generating processes. Friction converting mechanical energy into thermal energy is another ubiquitous source of heat. Electrical currents and electromagnetic radiation like visible light are common forms of heat generation. Chemical reactions and nuclear reactions release significant amounts of heat when energy stored in molecular bonds is converted. Geothermal energy taps into the internal heat of the Earth. Metabolic processes in living organisms also generate heat as a byproduct of biochemical reactions. By understanding the varied sources of heat found across nature, we gain a deeper appreciation for the thermodynamics that govern our universe.