Is Thermal Light Or Heat?
Thermal radiation, sometimes known as heat radiation, refers to electromagnetic waves emitted from the surface of an object due to the object’s temperature. It was first discovered in the 19th century by physicists who were studying heat and radiation.
All objects with a temperature above absolute zero emit thermal radiation. As an object becomes hotter, it emits more thermal radiation per unit surface area. Thermal radiation does not require a medium to travel through – it can propagate through a vacuum, unlike heat conduction or convection which require matter to transfer thermal energy.
The discovery of thermal radiation played an important role in the development of modern physics. It provided evidence supporting the concept that heat is a form of energy, and led to the new field of radiative heat transfer which studies how thermal radiation is emitted, absorbed, and scattered.
Properties of Thermal Radiation
Thermal radiation refers to electromagnetic waves emitted by matter that is above absolute zero temperature. All matter with a temperature radiates thermal energy in the form of electromagnetic waves. The wavelength range of thermal radiation spans from around 0.1 to 100 μm, which includes part of the infrared spectrum.
The key properties of thermal radiation that distinguish it from visible light include the wavelength range and the method of propagation. While visible light has wavelengths from 400-700 nm, thermal radiation’s longer wavelengths mean it has lower photon energies. This allows thermal radiation to be absorbed by matter and transfer heat. Unlike visible light which must have a medium to travel through, thermal radiation can propagate through a vacuum as electromagnetic waves.
Because all matter above absolute zero emits thermal radiation, the transfer of heat via radiation does not require direct contact between objects. Thermal energy propagates through space or any transparent medium as electromagnetic waves until being absorbed by matter. The amount of radiation emitted depends on the object’s temperature.
Difference Between Light and Heat
While the terms “light” and “heat” are often used interchangeably in everyday language, there are some important scientific distinctions between the two concepts. Light and heat represent different forms of electromagnetic radiation that have distinct properties and behaviors.
The key difference lies in wavelength. Visible light that humans can see has wavelengths from about 380 to 750 nanometers. On the other hand, thermal radiation, which we perceive as heat, has longer wavelengths between about 750 nm to 1 mm. This long-wavelength infrared radiation is sometimes called thermal radiation.
So while visible light and thermal radiation are both forms of electromagnetic waves, they occupy different positions on the electromagnetic spectrum. Light energy stimulates the retina of the eye, allowing us to see. Thermal energy from infrared radiation is absorbed by molecules, causing them to vibrate faster and producing the sensation of heat.
In summary, light is visible electromagnetic radiation, whereas heat results from infrared radiation. Their differences in wavelength impart unique characteristics even though they are closely related phenomena.
Blackbody Radiation
A blackbody is an idealized physical body that absorbs all electromagnetic radiation that falls onto it. No radiation passes through a blackbody and none is reflected. Real objects never act as perfect blackbodies, as they reflect some incident radiation. However, objects that approximate blackbody behavior are referred to as “gray bodies”.
According to Planck’s law, the spectral radiance of radiation emitted by a blackbody depends only on the frequency of radiation and the temperature of the blackbody. There is no dependence on the shape, material, or structure of the blackbody. At any given frequency, the spectral radiance increases as the temperature increases. The peak emission wavelength of a blackbody is inversely proportional to its temperature. Hotter blackbodies appear bluish-white while colder blackbodies appear red.
For a blackbody at room temperature, the peak wavelength is approximately 10 μm, in the far infrared. As the temperature increases to visible red heat (~700°C), orange heat (~1000°C), and finally blue-white heat (~6000°C), the peak wavelength decreases to the mid-infrared, near-infrared, and visible light ranges. The relationship between temperature and peak wavelength is described quantitatively by Wien’s displacement law.
Real-World Examples
Thermal radiation manifests in several common real-world examples that we regularly encounter:
Sunlight
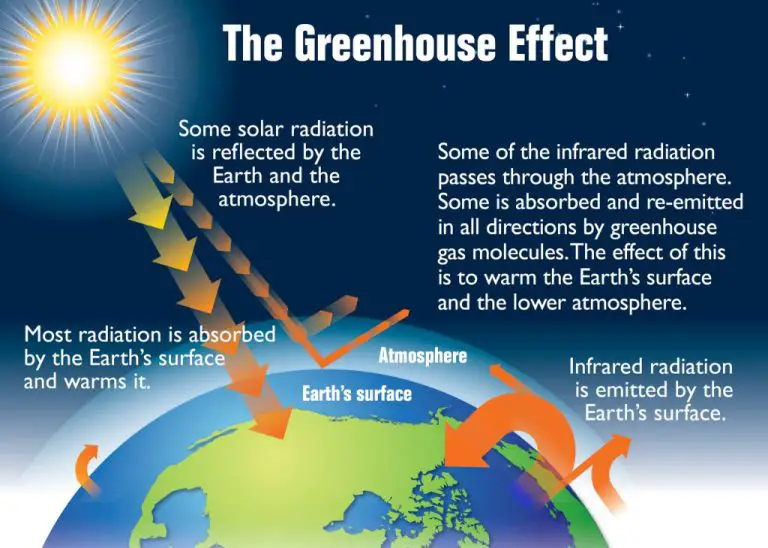
The sunlight that reaches the Earth’s surface contains infrared radiation or heat energy from the Sun. This radiation causes warming of objects on Earth. The infrared component of sunlight makes up around half of the total solar radiation spectrum.
Incandescent Bulbs
Traditional incandescent light bulbs produce light by heating a tungsten filament inside the bulb. This results in the emission of visible light, but also a significant amount of invisible infrared radiation or heat. Only about 10% of the energy emitted by an incandescent bulb is in the form of visible light.
Earth’s Infrared Emission
The Earth itself emits thermal infrared radiation as a result of absorbing incoming visible sunlight. The Earth re-radiates some of this absorbed energy as infrared heat radiation, resulting in an Earth energy budget that plays a key role in climate and weather.
Applications
Thermal radiation has many important applications in both science and technology. Some of the major applications are in night vision, thermal imaging, and spectroscopy.
Night vision devices detect infrared radiation to produce images in very low-light conditions. They amplify ambient infrared light and convert it into visible light. Night vision goggles and scopes allow people to see in the dark. Military, law enforcement, and rescue personnel often use night vision to operate at night.
Thermal imaging cameras detect thermal infrared radiation to see variations in temperature. They can produce images based on the differences in temperature of objects in the scene. Thermal imaging is used for military applications as well as firefighting, surveillance, and many industrial processes. It can help locate warm bodies and map heat loss from buildings.
Spectroscopy uses differences in thermal emission spectra to identify materials and their properties. By analyzing the selective radiation, absorption, and emission of thermal energy, spectroscopy can determine the atomic and molecular composition of substances. It is used widely in astronomy to analyze the chemical compositions of stars and planets.
Relationship to Thermodynamics
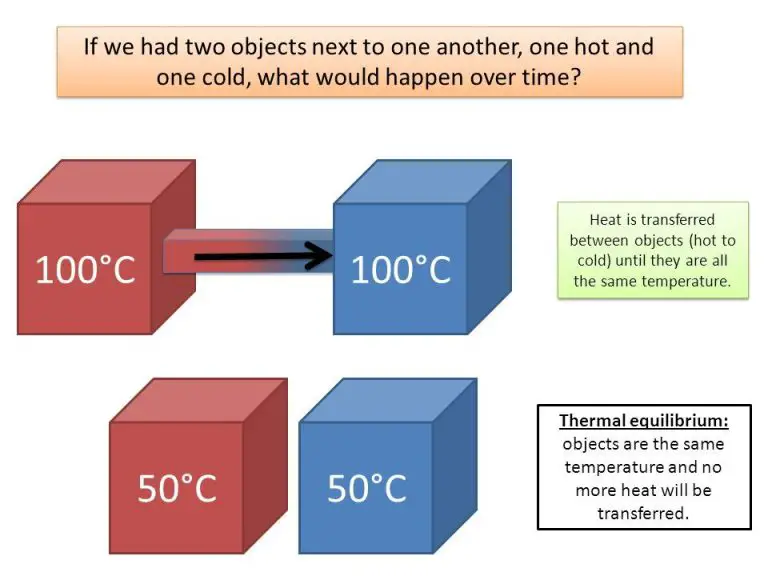
Thermal radiation is closely related to thermodynamics, the branch of physics that deals with heat and temperature. The laws of thermodynamics apply to thermal radiation and heat transfer through radiation. One of the primary ways that thermal radiation relates to thermodynamics is through radiative heat transfer. Radiative heat transfer refers to the exchange of heat in the form of electromagnetic radiation between objects at different temperatures. It allows thermal energy to be transmitted through a vacuum or transparent medium without the need for conduction or convection. This type of heat transfer follows the Stefan-Boltzmann law, which relates the power emitted from a blackbody to its temperature. Radiative heat transfer plays a key role in numerous engineering applications including heating and cooling systems, furnaces, rocket nozzles, and more.
Another connection between thermal radiation and thermodynamics involves the Carnot heat engine. This theoretical engine operates on a thermodynamic cycle between hot and cold temperature reservoirs. The efficiency of a Carnot engine depends on the difference between the hot and cold reservoir temperatures. A greater temperature difference allows more heat to be converted into useful work. Thermal radiation from the hot reservoir provides the heat input for driving the Carnot cycle. The radiation laws determine the amount of heat transfer possible based on the reservoir temperatures. Therefore, thermal radiation imposes fundamental limits on the maximum possible efficiency of heat engines as described by classical thermodynamics.
Effects on Matter
When thermal radiation interacts with matter, it can be absorbed, reflected, or emitted depending on the properties of the material. The way matter responds to thermal radiation depends on factors like the chemical composition, surface texture, and temperature of the material.
Absorption occurs when the photons that make up thermal radiation are taken into the material. The photons transfer their energy to the absorber’s molecules or atoms, increasing the internal energy and consequently the temperature of the material. Good absorbers of thermal radiation tend to be dull, dark surfaces like blacktop pavement or black paint.
Reflection takes place when the photons bounce off the surface of the material and do not get taken in. Shiny, light-colored surfaces like mirrors, aluminum foil, or snow reflect thermal radiation effectively and remain cooler than absorptive surfaces under the same conditions.
Emission happens when a material radiates thermal energy in the form of electromagnetic waves. All matter with a temperature above absolute zero emits thermal radiation, with hotter objects emitting more intensely than colder ones. Non-reflective black surfaces are the most efficient emitters.
Understanding the interplay between absorption, reflection, and emission of thermal radiation is important for designing and controlling the temperature of buildings, spacecraft, industrial processes, and more. Advances in “metamaterials” that can be engineered to exhibit custom absorption and emission properties open up many possibilities for future technologies.
Advances in Research
There are many exciting advances being made in thermal radiation research. Scientists are looking for new ways to better understand, measure, and utilize thermal radiation across a variety of fields.
One key area of focus is improving remote sensing technologies. By using thermal imaging and spectroscopy, researchers can gather detailed data about temperature and material properties from a distance. This has applications in everything from weather monitoring to medical imaging. New detectors and algorithms for processing thermal data are allowing for higher resolution images and video.
Researchers are also working to exploit thermal radiation for energy applications. From solar thermal devices to thermophotovoltaics, there are continued efforts to efficiently convert heat into electricity. Scientists are developing new selective emitters and nanostructures to better control thermal radiation for these technologies.
Additionally, there is increased interest around thermal metamaterials – engineered structures that interact with thermal radiation in unconventional ways. Metamaterials could enable thermal invisibility cloaking, radiation control, and other exotic effects. Though still in early stages, these metamaterials may open up new possibilities in thermal engineering.
While thermal radiation is a mature field, there are still many opportunities for transformative discoveries. With advanced materials, improved instrumentation, and computational modeling, researchers are pushing the boundaries of what’s possible with the thermal emission, absorption, and propagation of light.
Conclusion
Thermal radiation plays an important role in many areas of science and engineering. As we have seen, thermal radiation is electromagnetic radiation emitted by matter as a result of its temperature. It exhibits properties of both waves and particles. Thermal radiation transfers heat energy between objects, often without heating the medium in between. Understanding thermal radiation is key to designing efficient heating and cooling systems, modeling climate change, exploring the cosmos, and more.
While thermal radiation shares some properties with visible light, it is a distinct phenomenon. Thermal radiation encompasses a broad spectrum of wavelengths, both visible and invisible. All objects above absolute zero emit thermal radiation, with hotter objects emitting more intensely than colder ones. The relationship between temperature and thermal radiation is described by Planck’s Law.
Research into thermal radiation is ongoing, with new technologies being developed to harvest waste heat and convert it into usable energy. As our knowledge expands, so too will our ability to model and manipulate this ubiquitous form of energy transfer for the benefit of society.