What Type Of Energy Transfer Is Thermal Energy?
What is Thermal Energy?
Thermal energy refers to the energy that is transferred between objects or systems due to a temperature difference. It involves the motion of atoms and molecules – the higher the temperature, the faster the atoms and molecules vibrate and move. Thermal energy flows from a higher temperature object to a lower temperature object. For example, when you pour hot water into a cup, thermal energy flows from the hot water into the cooler cup, heating up the cup. Another example is heating up food in the microwave – the microwaves transfer thermal energy into the food, increasing the vibration of the food molecules and thus heating up the food.
We encounter transfers of thermal energy every day. Cooking, heating and cooling systems, car engines, and even our own bodies involve thermal energy being transferred from one object or system to another. Generally, thermal energy flows from hotter objects to colder objects until both reach the same intermediate temperature.
Forms of Thermal Energy Transfer
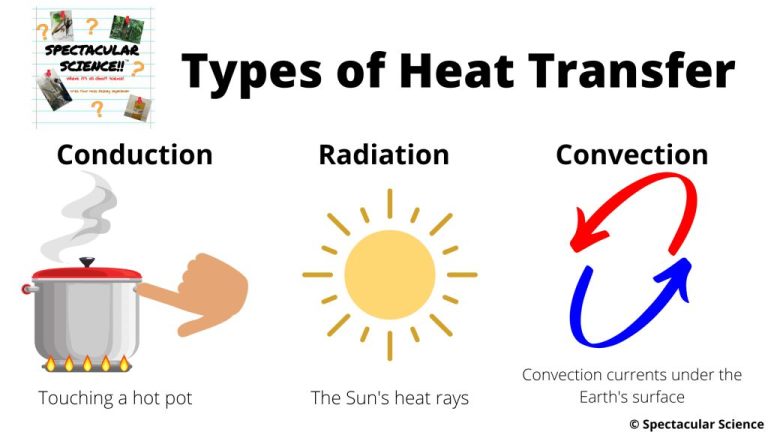
Thermal energy can be transferred between objects or regions through three main mechanisms: conduction, convection, and radiation.
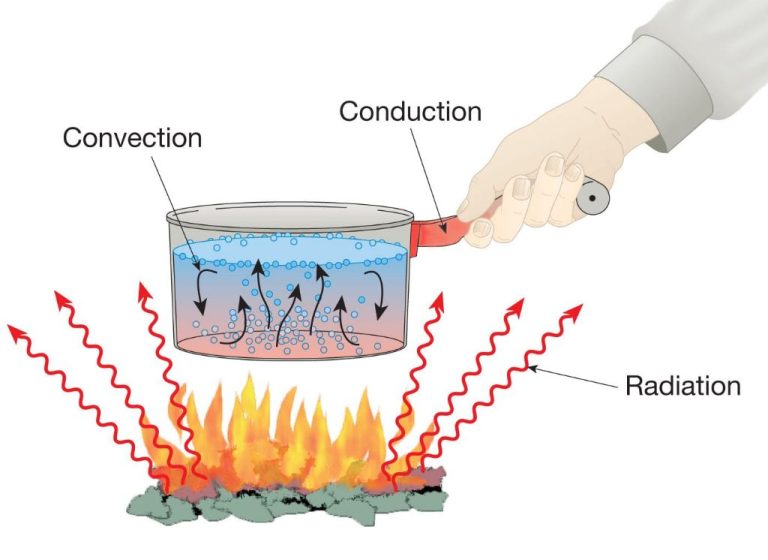
Conduction is the transfer of thermal energy between objects in direct physical contact. It occurs when heat flows from the hotter end to the colder end of an object or between objects in thermal contact, like a spoon warming up when placed in hot soup. Metals are good conductors of thermal energy.
Convection is the transfer of thermal energy by the motion of fluids. It occurs in liquids and gases. As the fluid is heated, it expands, becomes less dense, and rises. The cooler, denser portion then sinks to be heated. This circulation distributes the thermal energy. Examples include hot air rising off a radiator or the ocean’s convection currents.
Radiation is the transfer of thermal energy by electromagnetic waves directly through space. It does not require direct contact between source and destination. The rays emanate from a source and travel until they are absorbed by an object. An example is the warming sensation of standing in sunlight.
Conduction
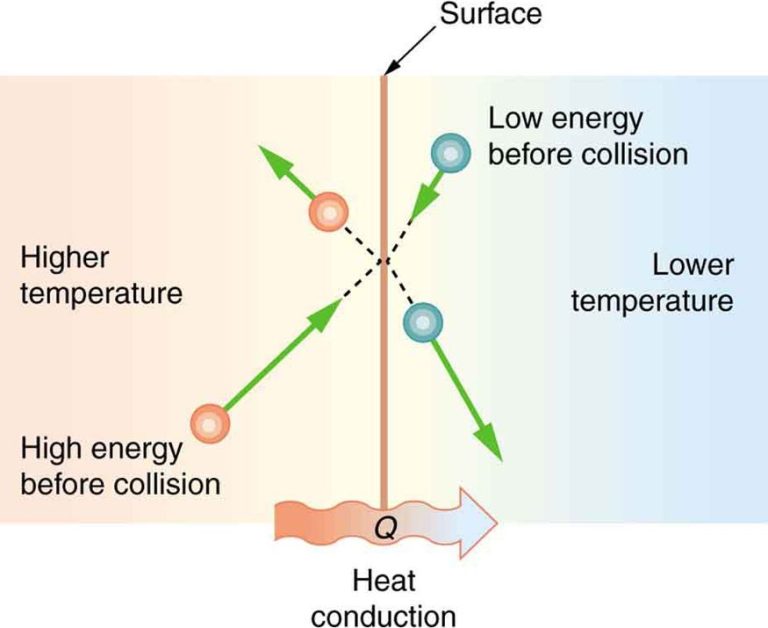
Conduction is a type of thermal energy transfer in which heat is directly transferred between particles of objects or substances that are touching. When there is a temperature gradient between two materials in contact with each other, heat will flow from the warmer material to the cooler one. Heat is transferred by the atoms and molecules vibrating and colliding with each other.
Conduction occurs most efficiently in solids since their particles are close together and fixed in place. Metals in particular are very good conductors of thermal energy because they contain free electrons that can transport thermal energy rapidly through the metal. Other good conductors are nonmetals like diamond, which has tightly bound carbon atoms.
Insulators have more weakly bound particles that vibrate and collide less, impeding the conduction of heat. Examples of good insulators are wood, plastic, rubber, and glass. Adding insulation reduces conduction and helps maintain desired temperatures.
Convection
Convection is a form of thermal energy transfer that involves the movement and circulation of fluids such as liquids and gases. When a fluid is heated, it expands, becomes less dense, and rises. The cooler, denser portion of the fluid then sinks to replace it, creating circulation. This movement and circulation allows thermal energy to be transferred through the fluid from areas of higher temperature to areas of lower temperature.
A common example of convection is the currents that form in water when heated. As water at the bottom of a pot gets heated by the stove, it expands, becomes less dense, and rises to the top. Meanwhile, the cooler water near the surface sinks down to replace it. This creates the currents that distribute heat through the pot.
Convection currents also occur in air and other gases. For example, when warm air rises from the heated ground, cooler air moves in to take its place. This allows heat to be distributed higher into the atmosphere. Convection occurs in many natural systems such as lakes, oceans, and the atmosphere. It is an important process for evening out temperatures in fluids.
Radiation
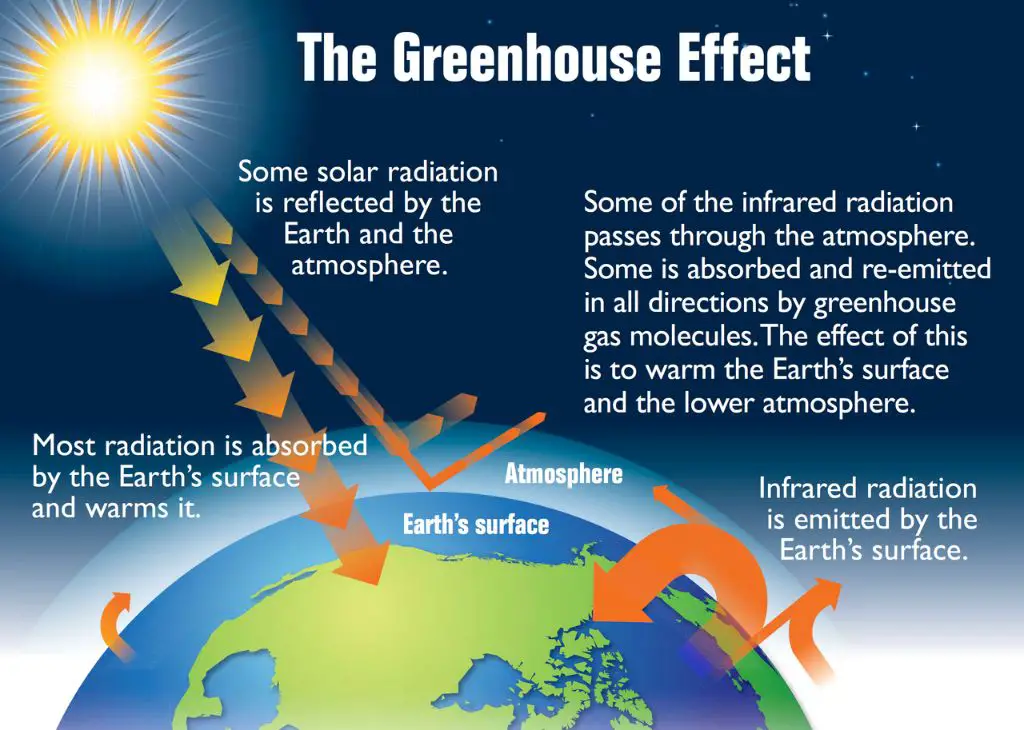
Radiation refers to the transfer of thermal energy via electromagnetic waves, or photons. Unlike conduction and convection, which require matter to transfer heat, radiation can transfer thermal energy across empty space. All objects constantly emit thermal radiation due to the temperature of their atoms and molecules. The hotter the object, the more thermal radiation it emits at shorter wavelengths. When thermal radiation strikes another object, it is absorbed and heats that object up. For example, the sun transfers thermal energy to the Earth predominantly through radiation, with the sun emitting thermal radiation that travels across empty space and is absorbed by the Earth, heating it up.
Examples of Thermal Energy Transfer
Thermal energy is constantly being transferred in the world around us through conduction, convection, and radiation.
A common example of conduction is the heating of a metal pot on a stove. The heat source transfers thermal energy into the molecules of the pot, causing them to vibrate faster and heat up the entire pot. The metal pot then conducts heat into the food inside of it.
Convection occurs in liquids and gases like the atmosphere. As molecules are heated, they move and carry energy from one place to another. For example, when water boils, the hotter, less dense water at the bottom of the pot rises while the cooler, denser water sinks – this movement distributes heat through convection.
Radiation does not rely on direct contact or movement of molecules. Instead, thermal energy is radiated as electromagnetic waves that can travel through a vacuum. For instance, the heat we feel from sunlight is caused by the radiation emitted by the sun propagating through space to the Earth’s surface.
Measuring Thermal Energy
Thermal energy is measured using different units depending on the system. Some common units used to measure thermal energy include:
-
Calories (cal) – A calorie is the amount of energy needed to raise the temperature of 1 gram of water by 1°C. Calorie is commonly used in nutrition and chemistry.
-
Joules (J) – A joule is a unit of energy in the International System of Units (SI). One joule is defined as the amount of work done when a force of 1 newton moves an object 1 meter. Joule is commonly used in physics.
-
British thermal units (BTU) – A BTU is the amount of energy needed to raise the temperature of 1 pound of water by 1°F. BTU is commonly used in engineering in the United States.
-
Therms – A therm is a unit of heat that equals 100,000 BTU. It is commonly used for natural gas billing in the United States.
Devices like calorimeters can precisely measure the change in thermal energy of a system. Thermometers and thermostats are common instruments used to measure temperature differences resulting from thermal energy transfer.
Importance/Applications
Thermal energy transfer is critical in many areas including nature, technology, and industry. In nature, thermal energy transfer impacts weather patterns, ocean currents, and animal adaptation and survival. The greenhouse effect, which warms the planet, relies on thermal radiation from the sun being absorbed by the earth’s surface and atmosphere. Without this transfer of thermal energy, the planet would be too cold for life.
Many technologies also depend on thermal energy transfer. Engines, power plants, refrigerators, air conditioners, and heaters all require forms of thermal energy transfer to operate. Conduction allows heat to transfer through metals in stoves, convection circulates air in heating and cooling systems, and radiation provides warmth from fireplaces and heaters. Thermal energy transfer principles enable these key inventions that control temperatures for human comfort and survival.
In industry, heat exchangers, boilers, condensers, radiators, and other equipment take advantage of thermal energy transfer. Manufacturing, chemical engineering, food processing, and other major industrial sectors rely on the controlled use of conduction, convection, and radiation. Understanding thermal energy transfer allows the design of efficient systems that can heat or cool materials for industrial production.
Overall, thermal energy transfer powers heating and cooling across nature, technology, and industry. It provides the warmth that sustains life, enables temperature-regulating devices, and drives industrial processes that require heating or cooling. Thermal energy transfer principles are applied widely to address essential needs in nature, engineering, and manufacturing.
Methods of Controlling Thermal Energy
There are various methods for controlling or manipulating the transfer of thermal energy. By understanding the three main mechanisms of heat transfer – conduction, convection, and radiation – we can find ways to speed up, slow down, or redirect the flow of heat.
Insulators are materials that resist conductive heat flow. They have low thermal conductivity and contain small air pockets that inhibit heat transfer. Examples of good thermal insulators include fiberglass, foamed plastics, wool, cotton, wood, and Styrofoam. Insulators can be used to slow heat transfer, for example in buildings and refrigerators.
Reflective barriers are materials that reflect radiation, such as polished metals and mirrors. They can redirect radiant heat, rather than absorbing it. Reflective insulation is used in building construction and even space blankets. Similarly, radiant barriers and low-emissivity coatings can help reduce heat transfer from thermal radiation.
Convection can be controlled by preventing or encouraging air circulation. Trapped air, such as in double-paned windows, reduces convection. Fans are used to speed up forced convection, as in computers. Convection currents can also be minimized by sealing gaps in insulation.
Other advanced methods for manipulating heat flow include heat pipes, thermal diodes, thermal storage systems, and regulating surface textures. Understanding the basic principles of thermal energy transfer allows us to control its flow for various applications.
Summary
Thermal energy refers to the internal energy within a system that is generated through the motion of atoms and molecules. The three main forms of thermal energy transfer are conduction, convection, and radiation. Conduction is the transfer of thermal energy through direct contact between materials. An example is a spoon heating up as it sits in a hot cup of soup. Convection is the transfer of thermal energy through the movement of liquids and gases. For instance, as water heats up, it circulates due to changes in density. Radiation is the emission of electromagnetic waves from all objects above absolute zero. An example is the heat we feel from sunlight.
Understanding how thermal energy transfers between objects and systems is crucial for many applications. It allows us to design efficient heating and cooling systems, insulate homes and appliances, cook food at precise temperatures, and convert energy into useful forms. With a solid grasp of conduction, convection, and radiation, we can better control thermal energy transfer for a wide range of purposes.