What Is Heat In Thermal?
Definition of Heat
Heat is a form of energy transfer between objects or systems due to a temperature difference. When two objects at different temperatures come into contact, heat flows spontaneously from the hotter object to the colder one until they reach thermal equilibrium. The transfer of energy continues until both objects are at the same temperature. Thus, heat represents the energy that is moving from one place to another as a result of temperature difference.
On a microscopic scale, heat arises from the random motion of atoms and molecules. The hotter an object is, the faster its atoms and molecules vibrate and move. As the rapidly moving particles in the hotter object collide with the slower ones in the colder object, energy is transferred, causing the colder particles to speed up. This energy transfer between atomic and molecular motion manifests itself macroscopically as a flow of heat.
Heat is directly related to temperature, but they are distinct concepts. Temperature measures the average kinetic energy of particles in a substance while heat is the total energy transferred between systems due to temperature difference. An object may have high temperature but low heat content if it has few particles. Thus, heat depends on the quantity of matter, while temperature does not.
Difference Between Heat and Temperature
Heat and temperature are related concepts, but they refer to different properties of a system. Temperature is a measure of the average kinetic energy of molecules in a system. It refers to the internal energy of each molecule based on its motion. Temperature does not depend on the amount or mass of material; even a small amount can have a high temperature if the molecular motion is very energetic.
Heat, on the other hand, refers to the total thermal energy contained within a system. It depends on both temperature and the amount of material present. Heat is a form of energy transfer between systems due to temperature differences. An object may have a lot of internal heat energy due to its mass and temperature, but limited temperature if the molecular motion is low.
For example, a bathtub of hot water has a high temperature and a lot of heat energy due to its volume and water temperature. A pot of boiling water on a stove may have a higher temperature but less heat energy overall due to its smaller mass.
How Heat is Transferred
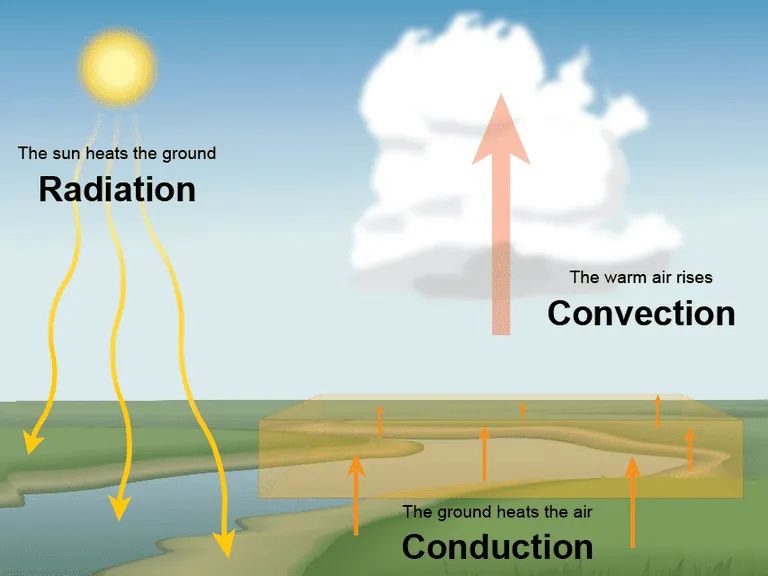
Heat transfers from one object or system to another in three main ways: conduction, convection, and radiation.
Conduction
Conduction is the transfer of heat between substances that are in direct contact with each other. It occurs when electrons from warmer molecules vibrate and collide with neighboring molecules, transferring kinetic energy. Metals are good conductors because they contain many free electrons.
Convection
Convection is the transfer of heat by the movement of currents within a fluid (liquid or gas). Hotter fluids rise and cooler fluids sink, causing circulation. Convection occurs in our atmosphere, oceans, and inside chimneys, cookware, and engines.
Radiation
Radiation is the transfer of heat energy by electromagnetic waves directly across space. No direct contact is needed. The sun emits radiant energy to the Earth by radiation. Other examples include heat from a fireplace reaching people nearby and heat radiating from hot asphalt.
Measuring Heat
Heat is measured in various units depending on the system being analyzed. Some common units used to measure heat include:
- Joules – The joule is the SI unit for energy and work. It is also commonly used to measure heat. A joule is defined as the amount of work done by a force of one newton moving an object one meter.
- Calories – The calorie was originally defined as the amount of heat needed to raise one gram of water by 1°C. Calories are commonly used to measure heat in chemical and biological systems.
- British Thermal Units (BTUs) – The BTU is a traditional unit of heat still widely used in the United States. One BTU is the amount of heat required to raise the temperature of one pound of water by 1°F.
In physics and engineering, the preferred unit is joules. But calories and BTUs are still commonly used, especially when discussing heat transfer in things like food, the human body, and buildings.
Specific Heat Capacity
Specific heat capacity refers to the amount of heat energy required to raise the temperature of a substance by 1 degree. Each substance has a different specific heat capacity, which is dependent on its molecular structure and composition.
The specific heat capacity is defined as the amount of heat energy required to raise the temperature of 1 gram of a substance by 1 degree Celsius. It is typically measured in J/g°C or cal/g°C. Substances like water have a relatively high specific heat capacity, requiring a lot of heat to raise its temperature. Metals on the other hand generally have low specific heat capacities.
The specific heat capacity is an intensive property, meaning it does not depend on the quantity or size of the material. It only depends on the type of material and its current state. Specific heat capacity is important for calculating the energy required for heating and cooling processes, and analyzing heat transfer between substances. It also explains why some materials heat up or cool down faster than others.
Heat Transfer Equations
Heat transfer equations describe how heat moves from one place to another. The main heat transfer equations are Fourier’s Law and Newton’s Law of Cooling.
Fourier’s Law relates heat flux to temperature gradient and thermal conductivity. Heat flux, q, is the rate of heat transfer per unit area. Temperature gradient, dT/dx, is how the temperature changes over distance. Thermal conductivity, k, is a property of the material. Fourier’s Law states:
q = -k * dT/dx
This shows that heat flux is proportional to the temperature gradient. The negative sign indicates that heat flows from high to low temperatures.
Newton’s Law of Cooling describes how an object loses heat to its surroundings. It states that the rate of heat loss is proportional to the temperature difference between the object and its environment. The equation is:
q = hA(T – Tsurr)
Where q is heat transfer rate, h is the heat transfer coefficient, A is the surface area of the object, T is the object’s temperature, and Tsurr is the surrounding temperature.
These fundamental equations allow calculation of heat transfer rates and temperatures in various applications. Engineers use them to design heat exchangers, insulations, electronics cooling, and more.
Effects of Heat
Heat has several important effects that show up in various applications and physical phenomena. Some of the main effects of heat are:
Thermal Expansion
When most materials are heated, they undergo thermal expansion meaning they increase in volume and length. This is because the increased kinetic energy from heat causes the atoms and molecules in the material to vibrate more intensely and push apart slightly. Metals tend to expand the most per degree temperature increase. The expansion can be predicted quantitatively using the material’s coefficient of thermal expansion.
Phase Changes
Heat can cause phase changes in matter, where a material transitions from one state (solid, liquid, gas) to another. For example, applying heat to solid ice will cause it to melt into liquid water once it reaches 0°C. Adding more heat past 100°C will cause the water to boil and turn into water vapor. Each of these phase changes requires heat energy, known as enthalpy of transformation, to break intermolecular bonds.
Chemical Reactions
Most chemical reactions are sped up by heat since the added thermal energy helps molecules overcome the activation barrier required for bonds to break and reform. Reactions involving combustion, such as burning wood or gasoline, are examples where heat plays a critical role in initiating and sustaining the reaction. The rate of chemical reactions approximately doubles with each 10°C increase in temperature.
Applications of Heat
Heat has numerous practical applications in everyday life and industry. Here are some of the most notable uses of heat:
Heating
Heat is used to warm buildings, water, and other substances. Heating systems like furnaces, boilers, and heat pumps rely on the transfer of heat to provide warmth. Controlling and optimizing heating is an important application of thermodynamics.
Engines
Heat engines like internal combustion engines and steam turbines convert heat into mechanical work. They use the flow of heat to drive pistons, turbines, or other devices. This allows heat to provide useful work in the form of electricity, locomotion, and more.
Cooking
Cooking relies extensively on the transfer of heat to food. Methods like baking, boiling, frying, etc. use heat to transform ingredients into tasty and safe meals. The principles of heat transfer underlie most cooking processes.
Industrial Processes
Many industrial processes depend on the application of heat. Examples include smelting metals, calcining limestone, drying paper, distilling alcohol, vulcanizing rubber, and countless more. Carefully managing heat is key for achieving efficient and high-quality results.
In summary, heat has become an indispensable part of human civilization. Mastering heat transfer and thermodynamics has enabled great advances in technology and productivity.
History of Understanding Heat

The understanding of heat has evolved over centuries as new theories and discoveries emerged. Some key developments include:
Caloric theory: The initial theory of heat stemmed from the caloric theory proposed in the 18th century. Caloric was seen as a self-repellant fluid that flowed from hotter objects to colder objects. Bodies with more caloric were hotter. This explained phenomena like heat flowing from fire to objects.
Heat as motion: In the 19th century, caloric theory was disproved as scientists like James Prescott Joule demonstrated that heat was related to the motion of molecules, not a distinct fluid. This showed heat could be created by motion or work. It laid the foundation of the mechanical theory of heat.
Thermodynamics development: Building on the idea of heat as motion, thermodynamics developed in the 19th century. Sadi Carnot analyzed heat engines, forging a link between heat and mechanical work. Rudolf Clausius introduced the concept of entropy. The laws of thermodynamics were formulated. Thermodynamics established a systematic framework to describe heat transfer and the relation between heat and other forms of energy.
Current Research on Heat
There are several interesting areas of research focused on improving efficiency and advancing thermal engineering techniques when it comes to heat:
Improving efficiency of heat transfer and thermal insulation in buildings – Researchers are developing new materials and techniques to maximize heat insulation and energy efficiency in building design. This includes high performance insulation, energy-efficient windows, and passive solar heating techniques.
Increasing efficiency of industrial heating processes – By using computational models and advanced materials, researchers are finding ways to optimize industrial furnaces, ovens and other heating processes to use less energy. Waste heat recovery systems are also being improved.
Advancing heat pumps and heat exchangers – Scientists are working on more efficient heat pump designs for heating and cooling, including adsorption heat pumps. Compact heat exchangers that transfer heat with less material are also being developed.
Thermoelectric materials – These materials can convert heat directly into electricity. Research on improving thermoelectric efficiency focuses on new materials, nanostructures, and device geometries.
Phase change materials – These substances use latent heat absorption/release at certain temperatures to store thermal energy. Research aims to find improved phase change materials for applications like solar power and spacecraft thermal control.
Thermal energy storage – New storage methods are being explored to more effectively capture waste heat or solar heat for later use. This includes advanced thermal energy storage devices and new phase change materials.