What Produces Magnetic Chemical And Heat Effects?
Magnetism, chemical reactions, and heat transfer are three fundamental concepts in physics and chemistry that explain how energy causes changes in matter. Magnetism involves the generation of magnetic fields and forces by electric currents and magnetic materials. Chemical reactions rearrange the structure of molecules through the breaking and forming of chemical bonds, often absorbing or releasing energy in the process. Heat transfer is the exchange of thermal energy between objects or systems due to temperature differences, causing changes in temperature over time.
This article will provide an in-depth explanation of how electric currents, permanent magnets, chemical bonds, and temperature changes produce the magnetic, chemical, and thermal effects that are ubiquitous throughout nature and technology. We will explore the physics and chemistry behind these phenomena and discuss some of their many applications.
Magnetic Effects
Magnetic effects are produced by the motion of electric charges. Moving electric charges generate magnetic fields, which exert forces on other moving charges. The most common source of magnetic fields is the flow of electrons in the form of an electric current.
According to Ampere’s law, an electric current produces a circular magnetic field around the current-carrying wire. The direction of the magnetic field can be determined by the right-hand rule. The strength of the magnetic field depends on the amount of current – a larger electric current produces a stronger magnetic field.
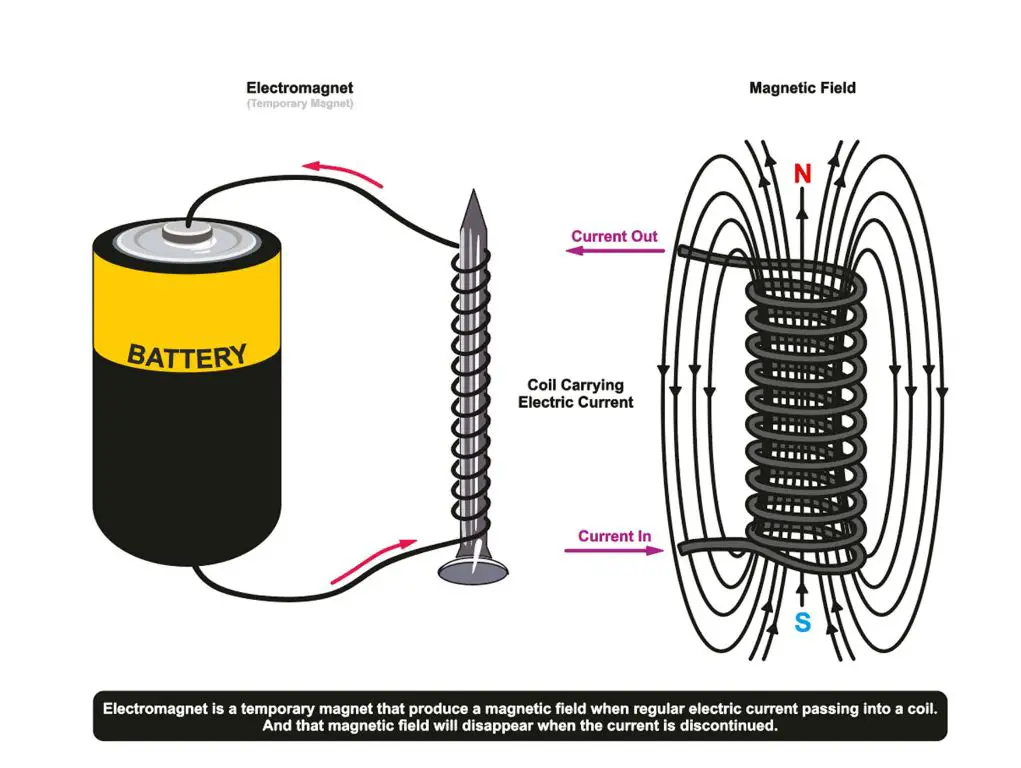
In electromagnets, the magnetic field is produced by a current-carrying coil of wire. The magnetic field gets stronger as more loops are added to the coil or a higher electric current flows through the coil. The magnetic field disappears when the current is turned off. Electromagnets are used in many electrical devices, engines, motors, and scientific instruments.
Electric Currents
Electric currents produce magnetic fields because they are composed of moving charges. As electrons flow through a wire, they constitute an electric current. These moving electrons have an intrinsic magnetic field associated with their electric charge in motion. The combined effect of all the electrons moving in the wire produces a magnetic field that encircles the wire.
The strength of the magnetic field produced is proportional to the amount of current flowing through the wire. So higher electric currents generate stronger magnetic fields, while lower currents create weaker fields. The direction of the magnetic field wraps around the wire perpendicular to the flow of the electrons. So the magnetic field essentially circles around the wire.
In summary, electric currents produce magnetic fields because they involve moving electric charges. The motion of the charges generates an associated magnetic field that wraps around the current-carrying wire. Stronger electric currents result in stronger magnetic fields.
Permanent Magnets
Some materials, like iron, can produce permanent magnetic fields. This is because the atoms in these materials have tiny magnetic fields produced by the electrons spinning around the atom’s nucleus. The spinning electrons act like tiny loops of electric current, which produce magnetic fields according to the right-hand rule of electromagnetism.
In most materials, the magnetic fields of the electrons point in random directions, canceling each other out. But in ferromagnetic materials like iron, the magnetic fields of the electrons align with each other, producing an overall magnetic field for the material. This alignment of electron spins happens because it allows the electrons to occupy a lower energy state.
The strength of the magnetic field in a permanent magnet depends on the number of aligned electron spins. The more spins aligned, the stronger the magnetic field. The alignment of spins can be strengthened by processes like heating and then cooling the material in a strong external magnetic field. This helps ‘train’ the electron spins into better alignment.
Chemical Effects
Chemical reactions involve rearranging the structure of molecules by making and breaking chemical bonds. Atoms within molecules are held together by chemical bonds, which involve the sharing or transfer of electrons between atoms. During a chemical reaction, old bonds are broken and new bonds are formed, resulting in different molecules being created.
For example, in an oxidation reaction like rust forming, iron atoms give electrons to oxygen atoms. The iron and oxygen atoms bond together to form iron oxide, which we see as rust. This rearrangement of atoms from iron metal to iron oxide is a chemical change produced by the magnetic effects of flowing electrons.
Chemical changes involve changes in the very makeup and organization of atoms within molecules. The flows and sharing of electrons between atoms drives this reorganization of molecular structure. So magnetic effects on the microscopic scale directly produce chemical changes that are observable on the macroscopic scale.
Bond Making/Breaking
Chemical bonds hold atoms together in molecules and compounds. When chemical bonds form between atoms, energy is released as heat. This exothermic process occurs because the total energy of the bonded atoms is lower than the combined energy of the separated atoms. Forming chemical bonds releases energy.
The opposite is true for breaking chemical bonds. Breaking the bonds between atoms requires energy input to overcome the attractive forces holding the atoms together. This endothermic process absorbs heat energy. The energy required to break a chemical bond is equal to the energy released when that bond initially formed.
Therefore, chemical reactions that involve making and breaking bonds will either release or absorb heat. Exothermic reactions like combustion occur when strong bonds are formed. Endothermic reactions that require heat input happen when bonds are broken. The energies involved in bond making and breaking produce observable thermal effects that can be harnessed in various applications.
Oxidation Reactions
Oxidation reactions involve the transfer of electrons from one substance to another and play a major role in producing energy. Some examples of oxidation reactions include:
Combustion: This is the reaction of a fuel with oxygen. For example, methane (CH4) reacts with oxygen gas (O2) to produce carbon dioxide (CO2) and water (H2O), giving off energy in the form of heat and light:
CH4 + 2O2 → CO2 + 2H2O + Energy
Rusting: When iron reacts with oxygen, it forms rust. This oxidation reaction requires both oxygen and water:
4Fe + 3O2 → 2Fe2O3
Food oxidation: The oxidation of nutrients like fats, proteins and carbohydrates in our cells produces energy for the body. A simple example is the oxidation of glucose:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
In each case, electrons are transferred from the fuel/iron/glucose to oxygen, resulting in energy production. Understanding oxidation is key to harnessing energy efficiently from chemical reactions.
Heat Effects
Heat can be produced and transferred through different methods. The three main mechanisms of heat transfer are conduction, convection, and radiation. Heat transfers from hot to cold regions, and will flow until there is equilibrium.
Conduction is the transfer of heat between objects in direct physical contact. Heat energy is transferred from molecules of higher energy to those of lower energy through interactions. Materials like metals are good conductors as their atomic structure allows heat to easily flow.
Convection involves heat transfer through a fluid like a gas or liquid. As the fluid is heated, it expands, becomes less dense, and rises. The cooler, denser fluid then moves to take its place – creating circulation. This process transfers heat around the system.
Thermal radiation is the emission of infrared radiation from an object due to its internal energy and temperature. It can travel through vacuums without direct contact. All objects radiate heat energy, with hotter objects emitting more intense radiation.
Understanding heat transfer mechanisms allows us to better control temperature in applications like heating and cooling systems. Factors like conduction, convection and radiation must be accounted for in the design of efficient systems.
Temperature Changes
Heat transfer causes variations in temperature, which is the measure of how hot or cold something is. The more energy transferred as heat, the more a material’s temperature will rise. There are three main ways that heat can be transferred:
Conduction is the transfer of heat through direct contact between two objects. A material like metal that easily conducts heat can rapidly raise the temperature of surrounding objects when in contact with them. For example, placing a pot on a hot stove allows conduction to quickly heat the pot and its contents.
Convection involves heat transfer via the movement of fluids or gases. Heated air or liquid rises while cooler air or liquid sinks. This circulation distributes thermal energy and changes temperatures. Convection ovens and convection currents in the atmosphere are examples of heat transfer by convection.
Radiation is the emission of electromagnetic waves that carry heat directly between objects without requiring contact or fluid transport. Radiant energy from the sun travels through space and raises temperatures on the Earth’s surface. Similarly, hot objects radiate heat in all directions, warming nearby cooler surfaces.
Understanding these heat transfer mechanisms is key to predicting and controlling temperature changes in a wide array of contexts, from materials processing to weather forecasting to cooking.
Applications
Magnetic, chemical, and heat effects have numerous practical applications in everyday life and industry. Here are some examples:
Healthcare – MRI machines use powerful magnets to visualize internal body structures. Chemical disinfectants sanitize surgical tools and hospital surfaces. Cauterizers apply heat to seal blood vessels during surgery.
Transportation – Electromagnets lift scrap metal and vehicles in scrapyards and factories. Gasoline and jet fuel undergo chemical combustion to propel cars, trucks, planes. Friction from brakes produces heat that slows vehicle wheels.
Electronics – Hard drives store data magnetically on spinning disks. Batteries rely on chemical reactions to generate electricity. Circuits and chips heat up during operation and require cooling fans or heat sinks.
Industry – Electromagnets separate iron from scrap metal. Chemical processes extract metals from ore. Furnaces and kilns apply intense heat to produce glass, ceramics, bricks, and more.
In summary, magnetic, chemical, and thermal effects are harnessed in numerous ways that make modern life and technology possible.