Is Thermal Energy Equal To Heat Energy?
Thermal energy and heat energy are related concepts in physics and thermodynamics. While they are closely linked, they refer to distinct forms of energy.
Thermal energy refers to the total internal kinetic and potential energy of all the molecules within an object. The faster the molecules vibrate and move, the more thermal energy they possess. Thermal energy is often referred to as the total internal energy of a system.
Heat energy, on the other hand, is the transfer of thermal energy between objects or systems that are at different temperatures. It refers specifically to the energy in transit from a hotter body to a colder one. Heat flows spontaneously from higher temperature to lower temperature objects.
While thermal energy refers to the total energy of motion within a system, heat energy denotes only the transferred portion of that internal energy. An object may contain thermal energy due to molecular motion, but there is no heat energy unless thermal energy is actually transferred between objects. Understanding this distinction is key to explaining concepts in thermodynamics.
Thermal Energy
Thermal energy refers to the total kinetic energy of all the molecules within an object. This energy comes from the vibrational motion of the atoms and molecules that make up matter. The faster the atoms and molecules vibrate, the more thermal energy they possess.
On a microscopic scale, increasing an object’s temperature speeds up the vibrational motion of its atoms and molecules. This increase in atomic and molecular motion corresponds to an increase in thermal energy. So temperature is a measure of the average kinetic energy of an object’s atoms and molecules. The higher the temperature, the more thermal energy is present.
Thermal energy is often referred to as heat. However, thermal energy is the total kinetic energy of all the molecules within an object, while heat refers to the transfer of thermal energy from one object or system to another due to temperature differences. So heat flow is driven by differences in thermal energy between objects in contact.
Heat Energy
Heat energy refers to the energy transferred between objects or systems due to a temperature difference. It flows from a higher temperature object to a lower temperature object. This energy transfer occurs at the atomic and molecular level as the atoms and molecules in the higher temperature object vibrate and move faster than those in the lower temperature object. These faster moving particles collide with the slower ones, passing kinetic energy to them.
Heat energy transfer can occur through three main mechanisms: conduction, convection, and radiation. In conduction, heat is transferred through direct molecular collisions, such as heating one end of a metal rod. Convection involves the bulk movement of heated fluid like air or water. Radiation transfers heat through electromagnetic waves and does not require a medium like air or water. Examples of radiative heat transfer include the warming of the Earth by the sun and infrared heaters.
Relationship
Thermal energy and heat energy are closely related concepts in physics. While they are often used interchangeably in everyday language, they have distinct scientific definitions.
Thermal energy refers to the total kinetic energy of all the atoms or molecules within a substance. This energy comes from the microscopic motion and vibrations of the particles. The faster the atoms or molecules vibrate and move, the more thermal energy they possess.
Heat energy, on the other hand, is the transfer of thermal energy between substances or systems that are at different temperatures. It flows from a hotter substance to a colder substance. So while thermal energy is contained within a substance, heat energy is in transit between substances as thermal energy is transferred.
So in summary, thermal energy is the stored kinetic energy of particles while heat is the movement of thermal energy from one system or object to another. Though related, they are distinctly different forms of energy in physics.
Measuring Thermal Energy
Thermal energy is measured in joules. A joule is a unit of energy defined as the work required to produce one watt of power for one second. In more simple terms, it is a measurement of the total kinetic energy of molecules within a substance.
To measure thermal energy, the total kinetic energy of all molecules in a system must be quantified. This is challenging to measure directly, so other proxy measurements are usually used instead.
Common techniques to measure thermal energy include:
- Measuring temperature – Temperature is directly proportional to the average kinetic energy of molecules.
- Measuring heat capacity – The amount of heat needed to raise a substance’s temperature by 1°C.
- Calorimetry – Measuring the heat flow into or out of a system as its temperature changes.
By using instruments such as thermometers and calorimeters, precise thermal energy values can be experimentally determined in joules.
Measuring Heat Energy
Heat energy is measured in joules, which is a unit of energy in the International System of Units (SI). A joule is defined as the amount of work done by a force of one newton moving an object one meter. In more practical terms, one joule is approximately the amount of energy needed to heat one gram of water by 0.24 degrees Celsius.
When an object or system gains heat energy, the motion of its molecules and atoms increases, which manifests as an increase in temperature. Measuring this temperature change, along with the mass and specific heat capacity of the substance, allows us to quantify the amount of heat energy transferred in joules.
Some examples of the amount of heat energy measured in joules:
- The energy needed to heat 1 liter of water from 15°C to 20°C is approximately 63,000 joules.
- One food Calorie (with capital C) is equal to 4,184 joules.
- A 60-watt light bulb operating for 1 minute emits 60 joules of heat energy.
So in summary, heat energy is measured in the fundamental unit of joules, by quantifying the temperature change in an object or system. This allows precise measurement of heat transfer in many scientific and engineering applications.
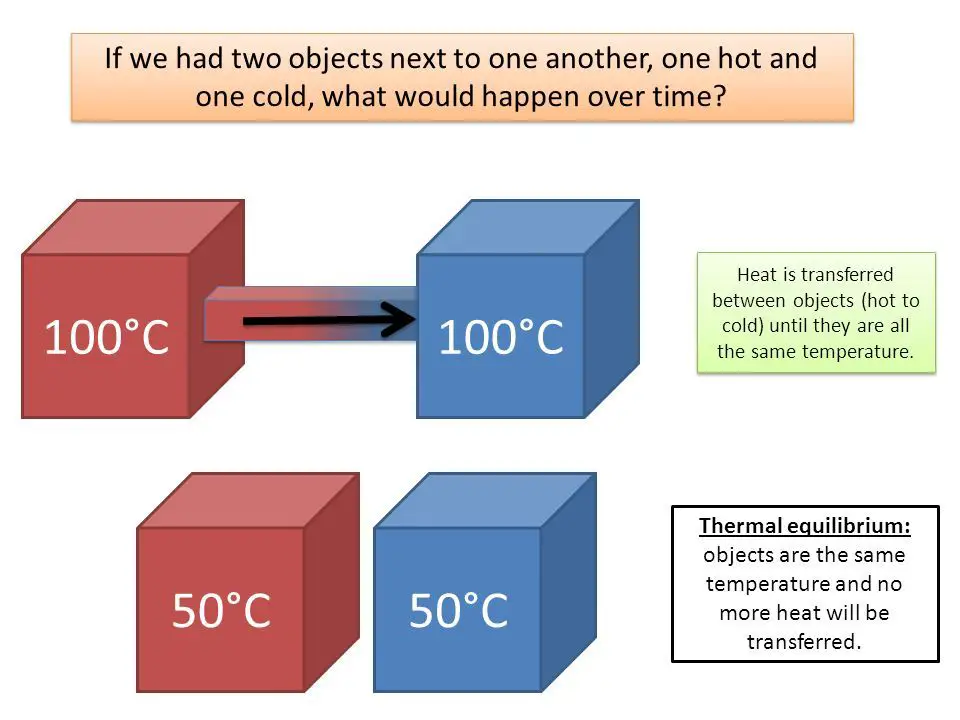
Thermal Equilibrium
Thermal equilibrium refers to when two or more objects or systems are in a state where there is no net transfer of heat between them. In other words, they have reached the same temperature and there is no driving force for heat to flow from one object or system to another. This happens when the systems are in direct contact with each other and have enough time to exchange energy until their temperatures equalize.
For example, when you pour hot water into a cold glass, net heat flows from the hot water to the colder glass, warming up the glass. Eventually, the temperatures of the water and glass reach the same value when they are in thermal equilibrium – no more heat flows between them. The water and glass have the same temperature at this point.
Thermal equilibrium is a key concept in thermodynamics. It represents the state where interacting objects or systems have no thermal gradient between them. Reaching thermal equilibrium is the driving force behind many thermodynamic processes and heat transfer interactions.
Examples
Two everyday examples of the relationship between thermal energy and heat energy are heating water and melting ice. When you boil water for tea, you are adding thermal energy in the form of heat from the stove burner. This raises the temperature of the water as the water absorbs the heat energy. The increased molecular motion from absorbing the heat is observed as a rise in temperature.
A similar process happens when melting ice into liquid water. Adding thermal energy in the form of heat causes the ice molecules to vibrate and move faster. As the ice absorbs this heat energy, some of the thermal energy goes into breaking the crystalline structure of the solid, allowing the molecules to move freely and transition from ice to liquid water. The additional thermal energy also increases the temperature of the liquid water above its freezing point.
In both examples, adding thermal energy in the form of heat results in observable changes in state and temperature. This demonstrates the direct relationship between heat energy and thermal energy.
Laws of Thermodynamics
The laws of thermodynamics govern the relationship between heat and other forms of energy. The three laws are:
First Law of Thermodynamics – This states that energy can neither be created nor destroyed, it can only be transformed from one form to another. For example, in a combustion engine, chemical energy from fuel is transformed into heat energy and kinetic energy.
Second Law of Thermodynamics – This states that heat flows spontaneously from a hotter to a colder body, but not in the reverse direction. Heat only flows one way until thermal equilibrium is reached.
Third Law of Thermodynamics – This states that the entropy (disorder) of a pure crystalline substance at absolute zero temperature is zero. In other words, a perfectly ordered system has no heat energy.
These fundamental laws describe how heat interacts with mechanical energy, chemical energy and other forms of energy in thermodynamic processes. The laws place constraints on how efficient heat engines can be engineered and impose limits on energy conversion processes.
Conclusion
In summary, while thermal energy and heat energy are related concepts, they have key differences. Thermal energy refers to the total kinetic and potential energy of all the molecules within a substance, which relates to the temperature. Heat energy specifically refers to the transfer of thermal energy between objects or systems. While temperature measures thermal energy at the molecular level, heat measures the flow and quantity of thermal energy being transferred. They follow the laws of thermodynamics which describe their relationship and flow. Understanding this distinction is important when analyzing thermodynamic processes.