Is Thermal The Same As Heat?
Defining Heat vs Thermal Energy
Heat and thermal energy are related concepts, but have distinct meanings in physics and thermodynamics. Heat refers specifically to the transfer of thermal energy between objects or systems. Thermal energy is the total kinetic and potential energy of all the molecules within an object.
When two objects at different temperatures come in contact, thermal energy transfers from the higher temperature object to the lower temperature object. This flow of thermal energy is called heat.
So while heat is energy in transit, thermal energy represents the total molecular energy contained within an object. Understanding the difference between these two terms is important for discussing thermodynamic processes.
Heat as Energy Transfer
Heat is the energy that transfers between two objects or systems due to a temperature difference. When two objects at different temperatures come into contact, energy flows from the object at a higher temperature to the object at a lower temperature until they reach thermal equilibrium.
Heat flows spontaneously from high to low temperatures. The greater the temperature difference, the faster heat flows. This transfer of heat energy continues until the objects have the same temperature. Once the temperatures are equal, the transfer of heat energy stops, as there is no longer a temperature difference driving the heat flow.
Unlike internal energy, heat is energy in transit. It flows between objects or systems due to interactions between particles and intermolecular forces. Heat itself is not stored in a system; it is the energy transfer taking place due to temperature differences between systems.
Thermal Energy and Temperature
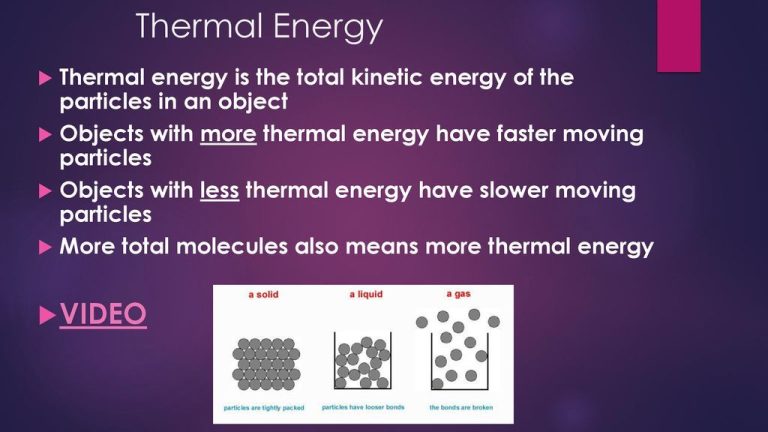
Thermal energy refers to the total kinetic energy of molecules within a substance. This kinetic energy is associated with the random motion of molecules. The higher the temperature of a substance, the greater the thermal energy since the molecules are moving faster on average.
Temperature is a measure of the average kinetic energy of molecules. As molecules speed up and move faster, the temperature rises. Thermal energy and temperature are closely related, but not quite the same thing. Thermal energy refers to the total kinetic energy of all the molecules, while temperature indicates the average energy on a per-molecule basis.
For example, a pot of water has high thermal energy compared to a cup of water because there are far more water molecules. However, both can be at the same temperature if the average kinetic energy per molecule is the same. Adding thermal energy to a substance increases its temperature. Removing thermal energy decreases the temperature.
Relationship Between Heat and Thermal Energy
Heat and thermal energy are closely related, but they are distinct concepts. Heat refers to the transfer of thermal energy between objects or systems. This transfer happens when there is a temperature difference. Thermal energy is the total internal kinetic and potential energy of all the molecules within an object.
Heat is energy in transit that flows from a high-temperature object to a lower-temperature object. When heat flows into an object, it increases the thermal energy of that object. This added thermal energy increases the temperature and kinetic energy of the molecules in that object. So heat transfer changes the thermal energy content of objects.
For example, when you boil water on a stove, the flame provides heat that transfers to the pot and water. This added heat increases the thermal energy of the water by increasing the water molecules’ kinetic energy. As more heat transfers over time, the thermal energy continues to rise until the water eventually reaches its boiling point temperature.
So in summary, heat flow causes changes to the thermal energy of objects. Adding heat increases thermal energy and temperature, while removing heat decreases thermal energy and temperature. The relationship is direct – heat transfer drives changes in thermal energy content.
Measuring Heat vs Thermal Energy
Heat and thermal energy are related, but distinct, concepts that require different units of measurement.
Heat is defined as the transfer of thermal energy between objects or systems. It refers specifically to the amount of thermal energy that is gained or lost in the process. Heat is measured in joules (J) in the SI system.
Thermal energy refers to the total internal kinetic energy of molecules within a substance. It is related to the temperature of the substance. Thermal energy can be quantified in two ways:
- In joules (J) – The total thermal energy contained in a system
- In kelvin (K) – The average kinetic energy of molecules, related to temperature
While heat and thermal energy are related, measuring them requires distinct units. Heat is a measure of energy transferred, while thermal energy quantifies the internal energy of a system.
Examples to Distinguish Heat and Thermal Energy
Heat and thermal energy are related but distinct concepts. Here are some examples to highlight their differences:
Imagine a pot of water on a stove. As the stove heats the pot, energy is transferred from the stove to the water, raising its temperature. This energy transfer is heat. The water molecules gain kinetic energy as they vibrate and move faster. This internal energy of the water is its thermal energy.
When you rub your hands together, the kinetic friction between your hands generates heat. This heat transfer increases the thermal energy and temperature of your hands. The faster you rub your hands, the more heat is produced.
An ice cube has thermal energy because its molecules vibrate even at low temperatures. If the ice cube is placed in a warm room, heat will transfer from the room to the ice, melting it. The ice gains more thermal energy as its temperature rises.
A paper towel can absorb a spill, soaking up the liquid. The towel does not change temperature, so no heat is transferred. However, the spill does transfer its thermal energy to the towel as it is absorbed.
In summary, heat involves energy transfer between objects, while thermal energy is the internal energy contained within an object itself. Distinguishing between the two is important for understanding thermodynamics.
Thermal Energy Transfer
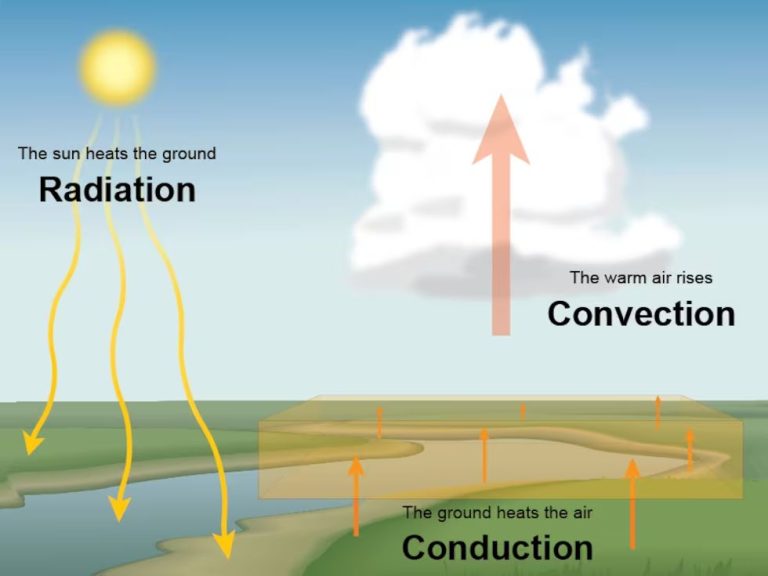
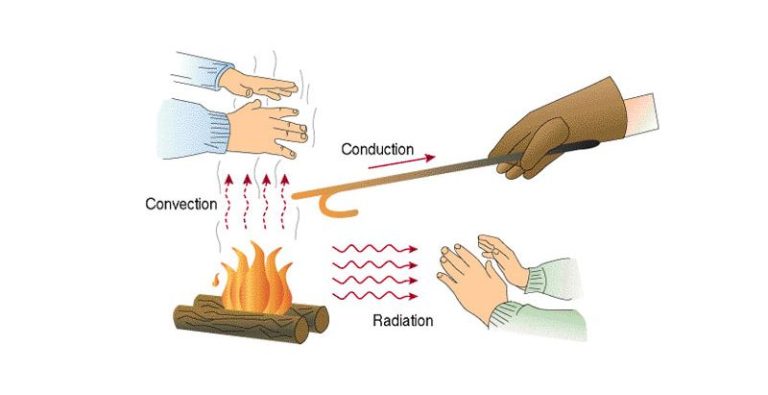
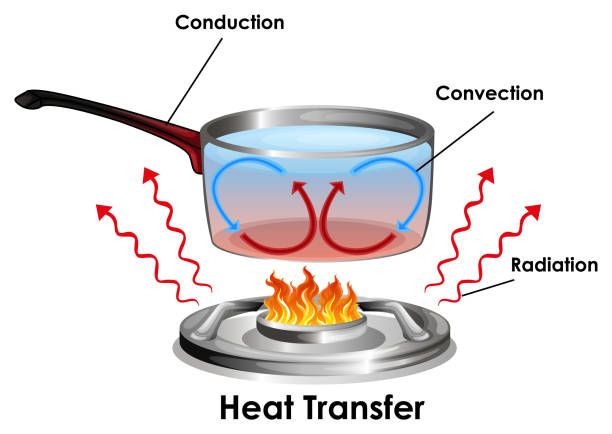
Thermal energy is transferred in three primary ways – conduction, convection, and radiation.
Conduction is the transfer of thermal energy between objects or substances that are in direct contact with each other. It occurs when heat flows from the hotter region to the colder region of an object or between two objects in contact. For example, a spoon placed in a hot cup of coffee will transfer heat from the coffee to the metal spoon through conduction.
Convection is the transfer of thermal energy by the movement of heated particles or substances from one place to another. It often involves a fluid or gas. For example, when water is heated on a stove, the hot water rises while the cooler water sinks – this movement of hot and cold water is convection. It also occurs in air, like when warm air rises and cooler air sinks.
Radiation is the transfer of thermal energy through electromagnetic waves or photons. No direct contact is required between the objects. The thermal energy is converted into electromagnetic radiation and can travel long distances. For example, the heat we feel from the sun reaches us through radiation.
Understanding how thermal energy transfers between objects and systems through conduction, convection and radiation is key to applications such as heating and cooling, energy generation, weather patterns, and more.
Applications
Thermal energy and heat principles have many practical applications in everyday life and technology:
Cooking: Applying heat to food is essential in cooking. As we heat food, the thermal energy in the pan or oven transfers to the food, raising its temperature and altering its properties through processes like maillard reactions and caramelization.
Engines: Heat and thermal energy are harnessed to power engines. In car engines, the controlled burning of fuel results in rapid heat release, increasing thermal energy which creates pressure to move the pistons. The thermal energy is converted into mechanical work.
Thermodynamics: The laws of thermodynamics govern how heat transfers between systems and how thermal energy can be converted into work. These fundamental laws are critical in the design of heat engines, refrigerators, and thermal systems.
Insulation: Insulation materials like fiberglass function by trapping air pockets, which are poor conductors of heat. This slows down heat transfer through the insulation, allowing it to maintain the thermal energy within a space.
Phase Change Materials: Substances like wax and water that undergo phase changes can absorb significant amounts of heat at constant temperature as they melt or boil. This enables practical applications as diverse as ice packs and thermal energy storage.
Review Key Differences
To summarize, while heat and thermal energy are related concepts, there are some key differences between the two:
-
Heat is the transfer of thermal energy between objects, while thermal energy is the total kinetic energy of molecules within an object.
-
Heat is a process, while thermal energy is a property of matter. Heat flows from one body to another, changing the thermal energy of both bodies.
-
Temperature measures the average thermal energy, while heat relates to the total amount of thermal energy transferred.
-
Heat has units of joules (J) or calories, while temperature is measured in units like degrees Celsius (°C), kelvin (K) or Fahrenheit (°F).
-
Heat can be measured with a calorimeter, tracking the change in thermal energy of objects. Temperature is measured with a thermometer.
-
Thermal energy depends on factors like mass, chemical composition, and molecular structure. Heat depends on temperature difference, mass, specific heat capacity.
In summary, heat is the transfer of energy due to temperature difference, while thermal energy is the total kinetic energy within an object that determines its temperature.
Conclusion
While heat and thermal are commonly used interchangeably, there are some key differences between the two scientific concepts. Heat refers to the transfer of thermal energy between objects due to a temperature difference. Thermal energy is the internal energy of an object that arises from the motion of its molecules and atoms. Heat always moves from higher temperature to lower temperature, while an object’s thermal energy depends on factors like its temperature, mass, and composition. Thermal energy can be quantified and measured, while heat is harder to measure directly.
To summarize, heat is a transfer process whereas thermal energy is a property of matter. Heat flows between objects, but objects contain thermal energy. While the terms are related, properly distinguishing between heat and thermal energy allows us to understand and describe important thermodynamic processes more precisely.
Through examples and analysis, we have clarified the nuanced differences between these two terms. While subtle, recognizing the distinct meanings of heat and thermal energy can remove ambiguity and improve scientific communication.