Does Thermal Energy Work?
What is Thermal Energy?
Thermal energy refers to the kinetic energy of molecules within a substance. It is often used interchangeably with the term heat energy. Molecules are always in motion, vibrating and rotating even in solids. The faster the molecules move, the more thermal energy they possess. Thermal energy is transferred from one substance to another through conduction, convection, or radiation. This transfer of thermal energy results in a temperature change.
The main distinction between heat and thermal energy is that heat is the transfer of thermal energy between substances, while thermal energy refers to the total kinetic energy of molecules within the substance itself. So heat is a process, while thermal energy is a property of matter. This internal energy is proportional to the temperature of the substance.
In summary, thermal energy arises from the kinetic energy of molecules and their motions. It can be transferred between substances through heat transfer, resulting in a rise or drop in temperature. Thermal energy is an intrinsic property of matter related to the motion and speed of its molecules.
Laws of Thermodynamics
The laws of thermodynamics govern the transfer of heat energy between systems. There are three primary laws that provide a foundational understanding of thermal energy:

The First Law of Thermodynamics states that energy cannot be created or destroyed, only converted from one form to another. In an isolated system, the amount of energy remains constant. This law is also known as the law of conservation of energy. It describes that thermal energy can transfer between systems, but the net change in energy of the universe is zero.
The Second Law of Thermodynamics states that in any closed system, the entropy (degree of disorder) increases over time. Entropy is a measure of randomness or chaos. This law explains that thermal energy tends to transfer from hotter to colder objects until equilibrium is reached. At equilibrium, entropy is at its maximum and no more energy transfer occurs.
The Third Law of Thermodynamics states that the entropy of a perfect crystal at absolute zero (0 Kelvin) is zero. Absolute zero is the theoretical minimum temperature where molecular motion stops. At this point, a system has reached perfect order and zero entropy. While absolute zero cannot be achieved practically, the third law provides a reference point.
Together, these laws describe the nature and limitations of thermal energy transfer. The first and second laws explain that while energy is conserved, it tends to spread out and become less useful over time. The third law provides the theoretical minimum entropy. These principles form the basis of thermodynamics.
Examples of Thermal Energy
Thermal energy is present in many aspects of everyday life. Here are some common examples of how thermal energy is utilized:
Body Heat
All living organisms produce heat as a byproduct of metabolic processes. Human body heat comes from cellular respiration and can be used to help maintain body temperature. Animals, including humans, also use body heat to warm shelters, eggs, and offspring.
Geothermal Energy
The Earth has immense amounts of thermal energy stored in hot rock and water deep underground. This geothermal energy can be harnessed in various ways, like using steam to turn turbines for electricity generation. Hot springs heated by geothermal energy are also used for bathing, heating, and industrial processes.
Combustion Engines
Internal combustion engines like those in cars and power plants burn fuel to convert chemical energy into thermal energy and mechanical work. The heat released from combustion reactions pushes pistons and turns crankshafts to power vehicles and machinery.
Nuclear/Fusion Reactions
Nuclear power plants split heavy atoms like uranium to release thermal energy, which is used to boil water, create steam, and spin turbines. Experimental fusion reactors use immense heat and pressure to fuse light atoms together, also generating large amounts of thermal power if the reactions can be sustained.
Thermal Energy Transfer
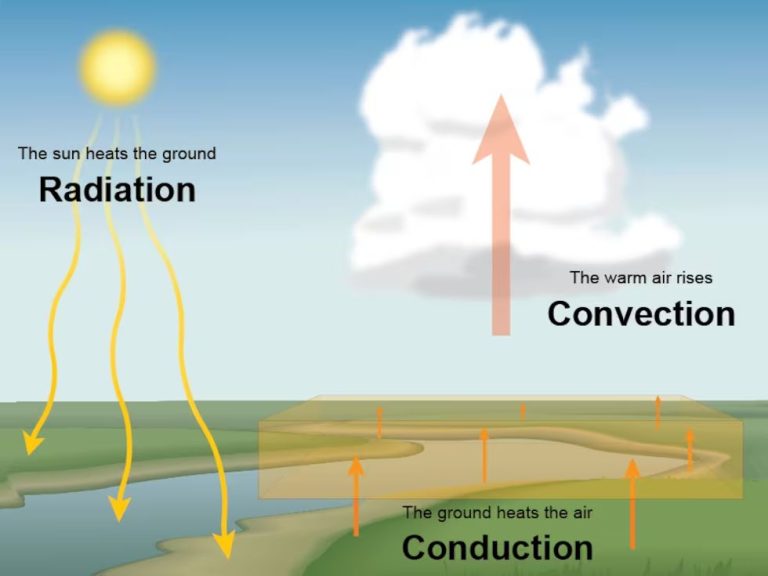
Heat, which is also called thermal energy, can be transferred between objects or locations through three main mechanisms: conduction, convection, and radiation. Understanding how thermal energy flows is crucial for many engineering and scientific applications.
Conduction
Conduction is the transfer of heat between objects that are in direct physical contact with each other. It occurs when heat flows from the hotter part of an object to the colder part, or from a hotter object to a colder object in contact with it. Metals are good conductors of thermal energy.
Convection
Convection is the transfer of heat by the actual flow of a liquid or gas. It occurs when warmer areas of a liquid or gas rise to cooler areas in the fluid, taking the heat with them. This causes circulation currents in the fluid. Convection plays an important role in weather patterns and ocean currents.
Radiation
Radiation is the transfer of heat via electromagnetic waves, or photons. It can transfer heat across empty space and does not rely on any contact between objects or a medium to transfer the heat. The sun transfers warmth to the Earth through radiation.
Phase Changes
Phase changes are also important methods of thermal energy transfer. When a substance changes from one phase to another, such as from solid to liquid, it absorbs or releases large amounts of energy. For example, when liquid water evaporates, it absorbs heat from its surroundings.
Measuring Thermal Energy
Thermal energy is typically measured using temperature scales and thermometers. The most common temperature scales are Fahrenheit, Celsius, and Kelvin. On these scales, temperature is a measure of the average kinetic energy of molecules in a substance. Thermometers measure temperature by responding to changes in thermal energy. Common types of thermometers include mercury, alcohol, digital and infrared. To measure the amount of thermal energy transferred between substances, a calorimeter can be used. A calorimeter is an insulated container filled with a known mass of water. By measuring the temperature change of the water after mixing with the heated or cooled substance, the thermal energy transferred can be calculated using the specific heat capacity.
There are a few key things to understand about measuring thermal energy:
- Temperature scales provide a standardized system to quantify thermal energy.
- Thermometers transduce temperature into an observable and measurable change.
- Calorimeters allow quantifying the amount of thermal energy transferred in a system.
- Insulation minimizes unwanted thermal energy transfers when taking measurements.
- Accuracy and precision in measurement are improved by calibration and reducing errors.
With the proper tools and techniques, thermal energy can be quantified and studied. Measuring thermal energy is critical for many scientific and engineering applications.
Storing Thermal Energy
Thermal energy storage allows heat or cold to be captured and used hours, days, or months later for heating and cooling needs. There are three main techniques for storing thermal energy:
Sensible Heat Storage
This involves raising or lowering the temperature of a storage material as energy is added or removed. Common storage mediums include water, rocks, ceramics, and molten salts. These materials experience a temperature change and store or release heat energy based on their heat capacity and the temperature differential. Sensible heat storage is simple and inexpensive but requires large volumes to store significant amounts of energy.
Latent Heat Storage
This makes use of materials that go through phase changes from solid to liquid or liquid to gas during the storage process. As heat is added or removed, the material absorbs or releases large amounts of latent heat without much temperature change. Common phase change materials include water/ice, paraffin waxes, and salt hydrates. Latent heat storage has a higher energy density than sensible heat storage.
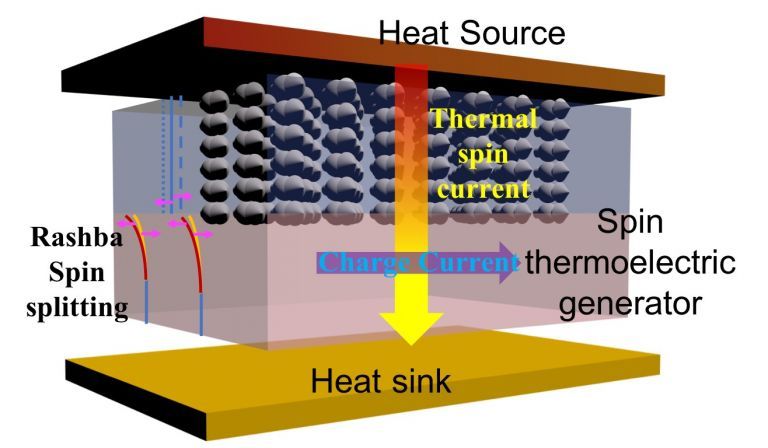
Thermoelectric Devices
Thermoelectric devices like Peltier coolers and thermocouples can also store thermal energy through electrical means. They use semiconductor materials to create a temperature differential and convert thermal energy directly into electricity or vice versa. However, their storage capacity tends to be low compared to other techniques.
Using Thermal Energy
Thermal energy has many practical applications in power generation, heating and cooling, industrial processes, and cooking.
One of the most common uses of thermal energy is in power generation. Many power plants burn fossil fuels like coal, oil, and natural gas to heat water into steam that spins turbines to generate electricity. Nuclear power plants use the thermal energy released from nuclear fission reactions to convert water into steam. Geothermal power plants tap into underground reservoirs of hot water to produce steam for electricity generation. Thermal solar power plants use mirrors or lenses to concentrate sunlight to heat fluids that drive steam turbines.
Heating and cooling systems also rely heavily on thermal energy. Furnaces, boilers, and heaters use the combustion of fuels or electricity to provide heat for homes, offices, and other buildings. Air conditioners and refrigerators move heat from one area to another using principles of thermodynamics and the compression and expansion of gases. Heat pumps can provide both heating and cooling by moving thermal energy in two directions.
Many industrial processes require the careful application of thermal energy. Smelting metals, manufacturing glass, drying materials, distilling alcohol, and making paper all depend on managing heat transfer at different temperatures. Commercial kitchens harness thermal energy for baking, boiling, grilling, frying, and other forms of cooking food.
In all of these examples, thermal energy plays a vital role in generating power, regulating temperatures, enabling manufacturing, and preparing food.
Thermal Energy Efficiency
Thermal energy efficiency focuses on minimizing heat loss and maximizing heat utilization. There are several main methods for improving efficiency:
Insulation
Insulation prevents heat transfer through building materials and systems. Common insulation types include fiberglass, mineral wool, cellulose, and foam. Properly insulating walls, roofs, floors, pipes, and HVAC systems reduces heating and cooling needs. Insulation standards have increased over time to save energy in new building construction. Retrofitting insulation in existing buildings also provides major efficiency gains.
Green Buildings
Green buildings utilize designs and technologies like natural ventilation, daylighting, passive solar heating, vegetation, and intelligent controls to minimize energy use. Efficient building envelopes, equipment, and practices reduce heating, cooling, and lighting loads. Green ratings like LEED and Energy Star certify buildings with proven energy savings.
Waste Heat Recovery
Waste heat from industrial processes, power generation, and HVAC systems can be captured and reused for heating or electricity generation. Technologies like heat exchangers, heat pumps, and Organic Rankine Cycles recover and utilize waste heat that would otherwise be released into the environment. This improves overall energy efficiency.
Cogeneration
Cogeneration facilities generate electricity and useful thermal energy simultaneously from a single fuel source, typically natural gas. The recovered heat gets used onsite for space heating, chilling, or industrial processes. Overall energy efficiency is enhanced by utilizing the heat that would otherwise be wasted. Cogeneration is also called combined heat and power (CHP).
Environmental Effects
The use of thermal energy can have several concerning environmental effects if not managed properly. Three major areas of concern are global warming, thermal pollution, and ozone layer depletion.
The burning of fossil fuels for thermal energy emits greenhouse gases like carbon dioxide into the atmosphere. These gases trap heat and contribute significantly to global warming and climate change. More renewable thermal energy sources like solar and geothermal that don’t emit greenhouse gases are needed to reduce this impact.
Thermal power plants require large volumes of water for cooling. This heated water is often discharged back into lakes, rivers or oceans, raising the temperature of the water in what is called thermal pollution. This can negatively impact marine ecosystems and biodiversity.
Certain industrial chemicals and refrigerants used for cooling rely on substances that deplete the ozone layer when released. The ozone layer protects the earth from harmful UV radiation. Its depletion increases health risks like skin cancer, harms plant growth, and contributes to climate change.
With proper regulation, technological improvements, and a shift to cleaner sources, the environmental effects of thermal energy can be significantly reduced. But it will require substantial effort and commitment to sustainable practices.
Future of Thermal Energy
The future of thermal energy looks bright as new renewable sources, fusion power, space travel, and new technologies emerge.
Renewable sources like solar and geothermal offer clean ways to harness thermal energy that don’t require burning fossil fuels. Solar thermal technology uses mirrors to concentrate sunlight and produce steam to turn turbines. Geothermal plants use underground reservoirs of hot water to power steam turbines on the surface. These renewable sources have potential to sustainably meet more of our thermal energy needs.
Fusion power holds promise as an almost limitless, emission-free energy source if the technology can be successfully harnessed. Fusion reactions like those that power the sun merge light atomic nuclei to form heavier elements, releasing massive amounts of energy. While technical barriers remain, progress is being made on fusion projects around the world.
Advances in space travel may rely on thermal energy. New thermal propulsion systems like nuclear thermal rockets could drastically improve the speed and efficiency of space travel by heating and expelling propellant material. This could enable interplanetary exploration and space missions that are currently beyond our reach.
Finally, new technologies like improved insulation, geoexchange systems, and tiny high-efficiency batteries could help us better conserve, manage and exploit thermal energy in the future. From smart thermostats to district heating systems, innovations will allow us to make the most of this ubiquitous energy source.