What Is Thermal Energy Found In Nature?
What is Thermal Energy?
Thermal energy refers to the total kinetic energy of molecules within an object or system. It is related to but distinct from temperature. While temperature measures the average kinetic energy of molecules, thermal energy describes the total internal energy of all these molecules combined.
Temperature indicates how hot or cold an object is, while thermal energy quantifies how much total energy it contains due to molecular motion. An object may have high thermal energy if it has a large mass at moderate temperature. A smaller object at the same temperature would have lower thermal energy, despite having the same temperature.
For example, a bathtub of hot water has more thermal energy than a cup of equally hot water because the bathtub contains more total water and molecules. The water in both containers would have the same temperature, but different thermal energy.
Understanding the difference between temperature and thermal energy is important when considering heating and cooling processes and heat transfer between objects. Thermal energy flows spontaneously from higher to lower energy states, while temperature seeks to equalize. In practical terms, thermal energy can be thought of as the amount of energy available to do work or transfer heat, while temperature reflects qualitative heat intensity.
Sources of Thermal Energy in Nature
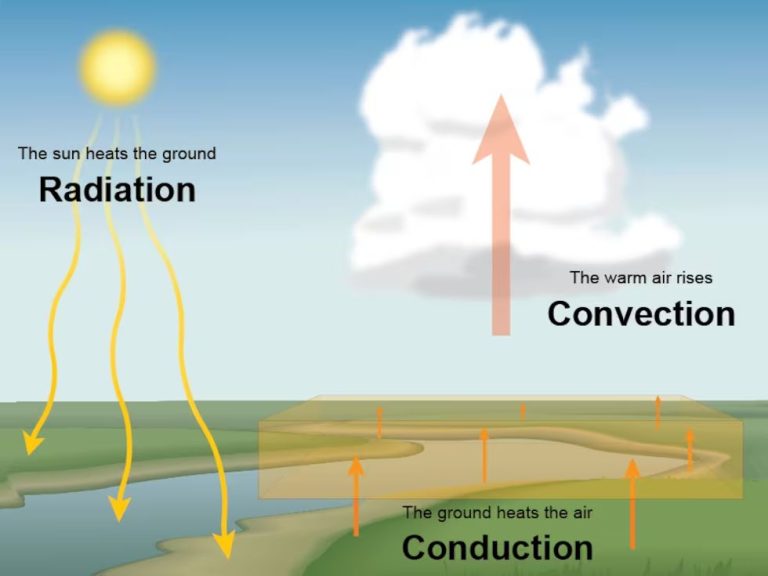
There are three main natural sources of thermal energy: the sun, geothermal energy from the earth’s core, and chemical reactions.
The sun is by far the largest source of thermal energy on Earth. The sun produces energy through nuclear fusion reactions, converting hydrogen into helium. This process releases enormous amounts of heat and light, which radiates outward in all directions. A tiny fraction of this solar energy reaches Earth and heats the planet’s surface and atmosphere.
Geothermal energy comes from the heat contained within the Earth’s core. The core is over 4,000 miles below the surface and contains molten rock and metal that can reach temperatures of 9,000°F. This heat gradually conducts outward, warming the surrounding mantle and crust. This geothermal energy appears at Earth’s surface as volcanoes and hot springs and can be tapped as a renewable energy source.
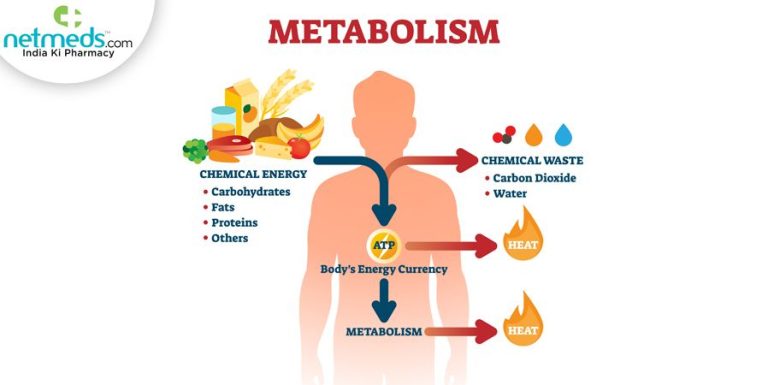
Chemical reactions can also produce thermal energy. Exothermic reactions like combustion, metabolism, and oxidation release heat as they break and form chemical bonds. These reactions power everything from wildfires to our own bodies. We harness the thermal energy from combustion to produce heat and electricity.
How the Sun Produces Thermal Energy
The sun produces an enormous amount of thermal energy through the process of nuclear fusion in its core. At extremely high temperatures and pressures, hydrogen atoms fuse together to form helium. This fusion reaction releases tremendous amounts of energy in the form of gamma radiation and thermal radiation.
The thermal radiation produced propagates outward from the solar core over a very long period of time. It travels through the different layers of the sun, gradually losing energy. By the time the radiation reaches the sun’s surface, it has cooled to about 5800K. This thermal radiation then streams freely into space in all directions. Only a tiny fraction of this radiation reaches Earth, over 150 million kilometers away, but this is still enough to warm our planet and drive weather systems, ocean currents, photosynthesis and all other natural processes relying on the sun’s thermal energy.
Using Solar Thermal Energy
Solar energy can be harnessed and converted into useful thermal energy for various applications:
Solar water heating – Solar collectors with dark absorber plates can heat up water that passes through tubes in the collectors. The heated water can then be used for residential, commercial and industrial applications.
Solar cookers – Parabolic mirrors can concentrate sunlight onto a cooking vessel. The concentrated sunlight heats up the vessel to cook food. Solar cookers are a sustainable cooking method requiring no fuels.
Passive solar heating – Buildings can be designed with large south-facing windows to let in sunlight. The sunlight directly heats up the building interior during the day. Strategies like thermal mass floors help retain and slowly release this heat overnight.
Geothermal Energy
Geothermal energy refers to heat energy generated and stored underneath the Earth’s surface. The molten core of the Earth, only tens of kilometres below the surface, can reach temperatures of over 4000°C. This immense heat emanates from radioactive decay in the Earth’s core. As this heat travels outwards towards the surface, much of it is dissipated; however, some thermal energy is conducted through the different layers of the Earth’s crust. In some areas where subsurface temperatures are sufficiently high, this geothermal energy can be harnessed at or near the Earth’s surface.
Natural manifestations of geothermal energy include hot springs, geysers, and fumaroles (steam and gas vents). Hot springs form when groundwater circulates to sufficient depth, gets heated, and then rises back up through fractures and pores in the rock. Geysers are similar to hot springs, but involve more constricted plumbing in the subsurface. This allows pressure to build up, leading to intermittent boiling and violent ejection of hot water and steam. Fumaroles are openings in the Earth’s surface from which hot gases emerge. All three of these represent geothermal energy being transported to the surface through natural processes.
Using Geothermal Energy
Geothermal energy has two main applications that take advantage of the constant temperatures underground: generating electricity with geothermal power plants, and heating and cooling buildings directly with geothermal heat pumps.
Geothermal Power Plants
Geothermal power plants are built over geothermal reservoirs with underground temperatures greater than 150°C. The hot underground water and steam are pumped to the surface to power steam turbines, which in turn drive generators that produce electricity. There are three types of geothermal power plants – dry steam, flash steam, and binary cycle. Dry steam plants use steam from the reservoir directly. Flash steam plants draw hot water from the reservoir, reduce the pressure to produce steam, then reinject the condensed water back underground. Binary cycle plants pass the hot water through heat exchangers to heat a secondary fluid which boils at a lower temperature than water. The vapor from the secondary fluid drives the turbines. Binary cycle plants emit very little greenhouse gases and can be built almost anywhere, but they are more expensive to build.
Geothermal Heating and Cooling
A geothermal heat pump system consists of pipes buried underground to move heat between the earth and buildings for space heating and cooling. In winter, the system uses the earth’s warmth to heat the building. In summer, the relatively constant temperature of the ground absorbs heat from the building. Geothermal systems are very efficient as the ground maintains a near constant temperature below the frost line, so very little energy is expended to heat or cool it. Direct geothermal heating systems pump water or steam from an underground reservoir directly into buildings. Though less efficient, they are cheaper to install and maintain.
Thermal Energy from Chemical Reactions
Chemical reactions are another major source of thermal energy in nature. Certain chemical reactions release energy in the form of heat, known as exothermic reactions. For example, when wood or other organic matter burns, the chemical reaction of oxidation releases stored chemical energy as heat. The oxidation reaction combines oxygen with other molecules, forming new compounds and generating thermal energy.
Oxidation reactions are very common in nature and everyday life. They not only explain how fire produces heat through burning, but also how animals derive energy from food through cellular respiration. On a geological scale, the slow oxidation of iron in the earth’s core is a source of geothermal energy. Exothermic chemical reactions constantly generate thermal energy throughout nature.
Chemical energy can also be purposefully harnessed and converted into thermal energy through designed exothermic reactions. For example, hand warmers utilize iron oxidation, while internal combustion engines ignite the combustion of fuel. Overall, chemical reactions provide a versatile way for natural and human systems to produce thermal energy.
Using Chemical Thermal Energy
Chemical reactions are a major source of thermal energy. Two examples of using chemical thermal energy are combustion engines and hand warmers.
Combustion engines like those in cars and airplanes rely on the combustion of fuel to release thermal energy which is then used to move the engine. Gasoline, diesel, and jet fuel contain chemical potential energy which is converted into kinetic energy through combustion reactions with oxygen. These types of engines have allowed humans to power automobiles, boats, trains and aircraft.
Hand warmers are small packets that produce heat through a chemical reaction. Often these contain iron powder, water, activated carbon, salt and wood dust. When the packet is exposed to air, an oxidation reaction takes place as the iron reacts with oxygen. This reaction gives off heat that can keep hands warm in cold weather conditions. Hand warmers are portable and provide a simple way to harness the thermal energy from a chemical reaction.
Measuring Thermal Energy
There are a couple main ways to measure thermal energy in nature and in systems we create. The two most common instruments used are thermometers and calorimeters.
Thermometers measure the temperature of a substance. Temperature is a measurement of the average kinetic energy of particles in a substance. As the thermal energy of a substance increases, the temperature rises. Thermometers allow us to quantify thermal energy in terms of degrees. Many types of thermometers exist, from mercury thermometers to digital thermometers. They can measure a wide range of temperatures.
Calorimeters are devices that measure the amount of heat absorbed or released during a chemical reaction or physical change. A simple calorimeter consists of an insulated container filled with a liquid, usually water. When a hot object is placed in the calorimeter, the temperature of the water rises. By measuring the temperature change and the mass of water, the amount of thermal energy transferred can be calculated using the specific heat capacity of water.
Calorimeters have many applications and allow us to quantify the thermal energy changes during chemical reactions and phase changes. This helps determine quantities like the specific heat capacity of unknown substances.
Importance of Thermal Energy
Thermal energy from natural sources plays a critical role in sustaining life on Earth. The moderate temperatures on our planet come from thermal energy, mainly from the Sun’s radiation. Without this constant input of thermal energy, the Earth’s average temperature would be about -18°C. At such frigid temperatures, liquid water could not exist, making life as we know it impossible.
In addition to maintaining life-sustaining conditions on Earth, thermal energy provides a major source of usable energy for human civilizations. People have harnessed natural sources of thermal energy for thousands of years, from simple campfires to modern nuclear power plants. Today, thermal energy accounts for the majority of worldwide energy consumption. Sources like combustion of fossil fuels, nuclear fission, and geothermal reservoirs produce heat that gets converted into mechanical or electrical energy. As societies move toward renewable energy, solar thermal and geothermal heat will likely play an increasing role in meeting humanity’s energy needs while reducing environmental impacts.