What Is Thermal Energy 7Th Grade?
What is Thermal Energy?
Thermal energy refers to the total kinetic energy and potential energy of molecules within a substance. It is often used interchangeably with the term heat, but they are not exactly the same thing. Thermal energy is the total energy while heat is the transfer of thermal energy from one object or system to another due to a temperature difference. Thermal energy is sometimes also referred to as internal energy.
Molecules are constantly vibrating and moving even when objects feel cold. The faster the molecules vibrate and move within an object, the more thermal energy it possesses. Temperature is a measure of the average molecular kinetic energy of a substance. So substances with a higher temperature generally have more thermal energy. However, temperature does not directly quantify the total thermal energy. Two objects at the same temperature may have different thermal energies based on their mass, chemical composition, phase (solid, liquid or gas), and other factors.
In summary, thermal energy refers to the total kinetic and potential energy of all the molecules within an object. Heat is the transfer of thermal energy from one object or system to another caused by a temperature difference. And temperature measures the average molecular kinetic energy which relates to but does not directly indicate the total thermal energy.
Forms of Thermal Energy
Thermal energy refers to the total internal energy of an object. This internal energy is associated with the random motion of molecules within the object. Thermal energy comes in several forms:
Heat is thermal energy that is transferred from one object or system to another due to a temperature difference. Heat flows from higher temperature objects to lower temperature objects until equilibrium is reached and the temperatures become equal.
Internal energy is the total kinetic and potential energy associated with all the molecules within an object. This includes both vibrational kinetic energy and potential energy stored in chemical bonds. Internal energy is the energy stored within a system.
Some examples of thermal energy include:
- The heat from a burning match
- The internal energy stored in gasoline molecules
- The warmth provided by a campfire
- The energy transfer that occurs when boiling water
Understanding the forms thermal energy can take is important when learning about thermodynamics and heat transfer.
How is Thermal Energy Transferred?
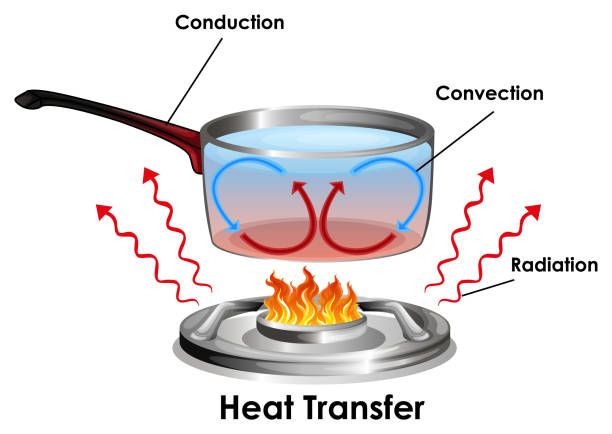
Thermal energy can be transferred in three main ways: conduction, convection, and radiation.
Conduction is the transfer of heat between objects that are in direct contact with each other. For example, when you place your hand on a hot stove, heat conducts from the stove into your hand. Metals are good conductors of thermal energy.
Convection is the transfer of heat by the movement of heated fluid or gas. For example, when water is heated on the stove, hot water at the bottom of the pot rises while cooler water sinks down to be heated. This movement distributes the heat through convection currents in the water.
Radiation is the transfer of heat in the form of electromagnetic waves. For example, the heat from the sun radiates to Earth across empty space. No direct contact is needed for heat transfer by radiation.
Examples of Thermal Energy Transfer
There are many examples of thermal energy transfer that we encounter in everyday life:
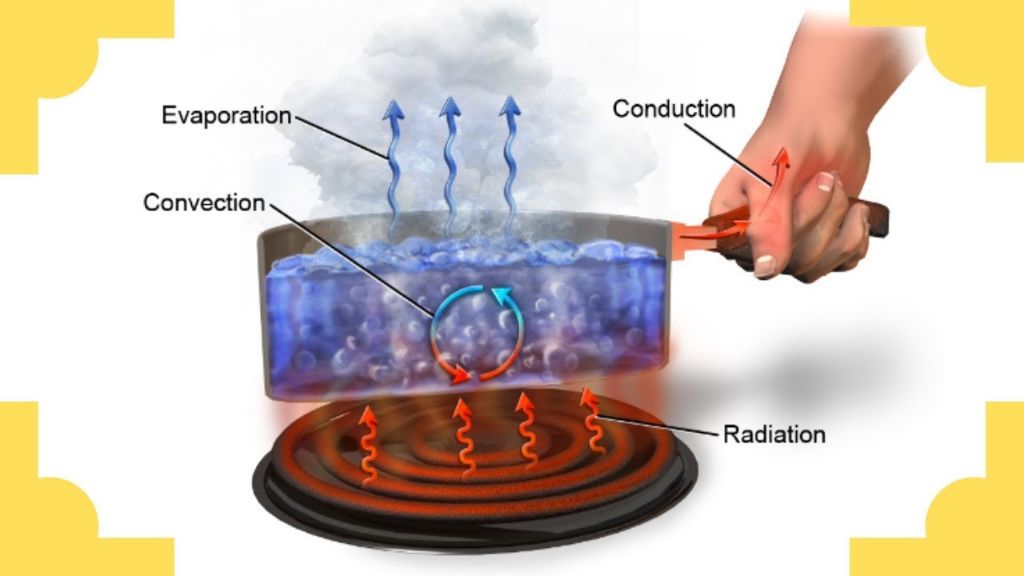
Touching a Hot Pan
When you touch a hot pan on the stove, thermal energy is transferred from the hot pan to your hand. The pan contains a high amount of thermal energy from heating over the stove burner. When you touch the pan, that thermal energy quickly moves from the pan to the skin of your hand, causing the sensation of heat. This is an example of thermal energy being transferred through direct contact and conduction.
Boiling Water
As water is heated in a pot on the stove, its molecules gain kinetic energy and begin to move faster. Once the water reaches its boiling point, bubbles form rapidly as the water transitions from a liquid to a gas phase. This phase change requires a significant input of thermal energy to break the bonds between water molecules. The thermal energy comes from the stove burner heating the pot. Boiling water demonstrates both conduction as the pot transfers heat to the water, and convection as the water circulates and heated water rises while cooler water sinks.
Earth Receiving Sunlight
The Earth receives a tremendous amount of thermal energy from the Sun in the form of electromagnetic radiation. The incoming solar radiation interacts with the atmosphere and surface of the Earth, heating our planet. The Earth then re-radiates some of this absorbed thermal energy back into space. The transfer of thermal energy from the Sun to the Earth through electromagnetic waves is an example of radiation as a thermal energy transfer mechanism.
Measuring Thermal Energy
There are a few main ways to measure thermal energy in science class. The most common is using thermometers to measure temperature. Thermometers measure the average kinetic energy of molecules in a substance. There are different temperature scales used including Fahrenheit, Celsius, and Kelvin. On the Fahrenheit scale, water freezes at 32°F and boils at 212°F. On the Celsius scale, water freezes at 0°C and boils at 100°C. The Kelvin scale is based on absolute zero, the coldest theoretically possible temperature. 0 Kelvin is -273.15°C.
Calorimeters can also be used to measure thermal energy transfer during phase changes. For example, when melting ice the thermal energy absorbed as the ice melts can be measured by the temperature change of the surrounding water in a calorimeter. Calorimeters have an inner cup to hold the sample substance and an outer cup to hold the surrounding liquid. Thermometers measure the starting and final temperatures, allowing calculation of the energy transferred.
Other devices like thermocouples can also measure differences in temperature electronically. Overall, thermometers and calorimeters allow measurement of thermal energy changes through tracking temperature changes.
Thermal Energy and Phase Changes
Thermal energy is responsible for phase changes, which are physical changes between solid, liquid, and gas states of matter. The main phase changes related to thermal energy are melting, freezing, evaporation, and condensation.
Melting occurs when a solid absorbs enough thermal energy to turn into a liquid. For example, ice melts when it absorbs thermal energy from the surrounding environment and changes from a solid to a liquid (water). The melting point is the temperature at which a substance changes from solid to liquid.
Freezing is the opposite process – it occurs when a liquid releases enough thermal energy to turn into a solid. For example, water freezes into ice when it releases thermal energy into the surrounding environment. The freezing point is the temperature at which a substance changes from liquid to solid.
Evaporation happens when a liquid absorbs enough thermal energy to turn into a gas. For example, water evaporates from a pond or puddle, turning into water vapor. The evaporation point is the temperature at which a liquid turns into a gas.
Condensation is the reverse process – it occurs when a gas releases thermal energy and turns back into a liquid. For example, water vapor in the air condenses into liquid water droplets on a cold surface like a window pane. The condensation point is the temperature at which a gas turns into a liquid.
In summary, thermal energy drives phase changes between solid, liquid, and gas states through melting, freezing, evaporation, and condensation. The absorption or release of thermal energy causes physical changes at specific temperatures.
Thermal Energy Storage and Transfer
Thermal energy refers to the total energy of motion in the particles that make up an object. This motion includes the vibration and rotation of atoms and molecules within substances. When an object is heated, its atoms and molecules move and vibrate faster, increasing its thermal energy.
The amount of thermal energy an object has depends on its mass, material, and temperature. Objects with more mass can store more thermal energy. Different materials also hold different amounts of thermal energy at the same temperature. This property is called specific heat capacity. Substances like metals have a low specific heat capacity, while water has a very high capacity to store thermal energy.
Thermal energy transfers between objects through conduction, convection, and radiation:
- Conduction is the transfer of thermal energy between objects in direct contact with each other, like a pot on a stove.
- Convection is the transfer of thermal energy by the movement of heated fluid or gas, like hot air rising or water boiling.
- Radiation is the transfer of thermal energy by electromagnetic waves, like the heat from the sun warming the Earth.
When an object is heated, increasing its thermal energy, the extra energy is gradually transferred to cooler surroundings over time. Conversely, a cold object absorbing thermal energy will warm up. The rates of heating and cooling depend on the temperature difference, properties like specific heat capacity, and the types of thermal energy transfer occurring.
Conduction Examples
Conduction is the transfer of thermal energy between objects that are in direct contact with each other. Metals are very good conductors of thermal energy, while non-metals like wood, plastic, and rubber do not conduct thermal energy well.
A common example of conduction is touching a hot pan on the stove. The thermal energy quickly transfers from the hot pan to your hand. Metals like the pan conduct heat easily, while non-metals like the plastic handle do not.
Another example is when you put a metal spoon in a hot cup of soup. The metal spoon quickly gets hot as the thermal energy transfers from the hot soup to the spoon through conduction. A wooden or plastic spoon would take much longer to heat up.
In cold weather, metal objects feel much colder than non-metals because they conduct heat away from your hand more rapidly. A metal railing outside will feel colder than a wooden railing at the same temperature.
Metals are used for cooking pots and pans specifically because they readily conduct thermal energy for quick and even heating. Non-metal materials like wood or plastic would not heat up as effectively on the stove.
Convection Examples
Convection is the transfer of heat by the movement of heated particles within a fluid. Some common examples of convection include:
Hot Air Rising
As air is heated by a heat source like the sun, it becomes less dense and rises. The cooler, denser air then sinks to take its place, creating a circular flow called a convection current. We feel this when warm air from a heater rises to the ceiling while cool air drops to the floor. Convection currents in the atmosphere create wind patterns like sea breezes.
Ocean Currents
The sun heats equatorial ocean water more than polar water, making that water less dense so it rises and cooler water sinks, driving ocean currents. Warm currents like the Gulf Stream carry heat from equatorial regions toward the poles, warming Northern climates.
Mantle Convection
Heat from Earth’s core makes the solid mantle rock slowly churn and move, driving plate tectonics. Hot mantle material rises at mid-ocean ridges while cooler slabs sink at subduction zones where oceanic plates are pushed under continental plates. This very slow convection of the mantle over millions of years shapes the surface of the Earth.
Radiation Examples
Radiation is the transfer of heat energy by electromagnetic waves. This is the only form of heat transfer that does not rely on particle collision. Some common examples of thermal radiation include:
- The sun warming the Earth – The sun produces energy from nuclear fusion reactions at its core. This energy is emitted in the form of electromagnetic radiation, which travels the 150 million kilometers from the sun to Earth. The Earth absorbs a portion of this radiation, heating the atmosphere, oceans, and land.
- Campfire – The burning wood and embers in a campfire emit thermal radiation that you feel as the warmth coming off the fire. The electromagnetic waves excite the molecules in your skin, causing the heat sensation.
- Glowing metal – As metal is heated to high temperatures, it begins to emit thermal radiation usually visible as a red, orange, or yellow glow. The color indicates the temperature of the metal.
- Incandescent light bulb – Traditional incandescent bulbs produce light via a thin tungsten filament heated to high temperature by electrical current. The hot filament emits thermal radiation, mostly in the visible light spectrum, which illuminates the surrounding area.
In these examples, thermal energy is transferred from one object to another over a distance by the radiation of electromagnetic waves. No direct contact between the objects is required for the heat transfer to occur.