Does Temperature Count As Energy?
What is Temperature?
Temperature is a measure of the average kinetic energy of molecules within an object or system. It refers to how hot or cold something is based on the motion and vibrations of its atoms and molecules. Temperature measures the internal energy present within matter that gets transferred between objects as heat. It quantifies the thermal energy contained within a substance.
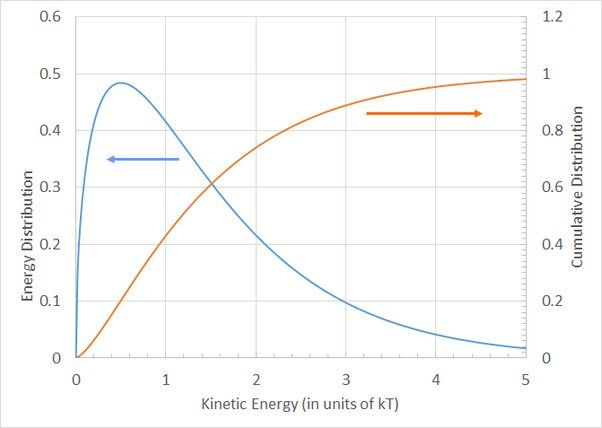
Temperature indicates the speed at which atoms and molecules vibrate and move. Higher temperatures mean the particles have more kinetic energy and move faster, while lower temperatures mean they have less kinetic energy and move slower. Temperature depends on the mass and types of atoms or molecules as well as the forces between them. It does not measure the total quantity of thermal energy, only the average energy on a per atom/molecule basis.
Relationship Between Temperature and Energy
While temperature itself does not constitute energy, it is closely related to energy at the molecular level. Specifically, temperature is proportional to the average kinetic energy of molecules within a substance. Kinetic energy is the energy associated with motion. At higher temperatures, molecules have greater average kinetic energy and move faster. At lower temperatures, molecules have lower average kinetic energy and move more slowly. For example, as water is heated from a liquid to a gas, its molecules speed up dramatically, indicating a large increase in kinetic energy.
This relationship allows temperature to be an indicator of the kinetic energy available in a system. Systems at higher temperatures tend to have more capacity to do work and transfer heat energy. However, temperature itself only describes the average kinetic energy, not the total kinetic energy or any other forms of internal energy within a substance. So temperature correlates with but does not directly quantify the energy of a system.
Temperature is Not Energy Itself
While temperature relates to energy, it is important to understand that temperature itself is not a form of energy. Temperature is a measure of the average kinetic energy of particles in matter. Kinetic energy is the energy particles have due to their motion.
For example, the atoms and molecules that make up a hot object have more kinetic energy and move faster on average than the atoms and molecules in a colder object. However, temperature does not directly quantify this kinetic energy. Rather, it describes the system’s “hotness” or “coldness” relative to a standard.
Temperature is an intensive property, meaning it does not depend on the size or amount of matter present. Energy is an extensive property, meaning the total energy increases with the amount of matter. So doubling the volume of a substance doubles its total energy, but not its temperature.
While temperature relates to the kinetic energy of particles, they are fundamentally different concepts. Kinetic energy is the actual energy present, while temperature is an indirect measure and indicator of that energy.
Heat vs. Temperature
Heat and temperature are often used interchangeably in everyday language, but they are distinct scientific concepts. Heat refers to the transfer of thermal energy between objects or systems, while temperature measures the average kinetic energy of molecules and atoms.
When two objects at different temperatures come into contact, heat flows from the warmer object to the cooler one until they reach thermal equilibrium. This heat transfer occurs because of interactions between molecules and atoms in the objects. Higher temperature means greater average kinetic energy of particles.
Temperature does not transfer between objects like heat does. It is an intrinsic property of a system that indicates the intensity of molecular and atomic motion. Temperature can be precisely measured with thermometers and other instruments. In contrast, heat is challenging to quantify directly. The amount of heat transferred can be calculated from the temperature change of an object.
Understanding the nuanced difference between heat and temperature is important in thermodynamics and many applications like HVAC systems, weather forecasting, chemical reactions, and more. While everyday language may use the terms interchangeably, scientifically they have distinct meanings related to thermal physics.
Temperature and Thermal Energy
While temperature and thermal energy are related, they refer to different physical properties. Temperature measures the average kinetic energy of molecules and atoms in a material. Thermal energy, also called heat energy, depends on the temperature, mass, and type of material.
Materials with a higher temperature contain particles moving faster on average, and thus possess more thermal energy. Heating increases molecular motion and kinetic energy, resulting in a higher temperature and greater thermal energy. Cooling reduces motion, lowering temperature and thermal energy.
For a given temperature, larger masses contain more atoms and molecules, so they possess more total thermal energy. Also, different materials with the same temperature may contain different thermal energy due to differences in molecular structure and composition.
In summary, while temperature provides a measurement of molecular kinetic energy, the total thermal energy depends on additional factors like mass and material type. Higher temperature materials possess more thermal energy, but thermal energy also scales with mass and varies across substances.
Measuring Temperature
Temperature is measured using various temperature scales, with the most common being Celsius, Fahrenheit, and Kelvin. Each scale is based on fixed reference points, allowing us to quantify and compare temperatures.
The Celsius scale sets 0°C at the freezing point of water and 100°C at the boiling point of water. Temperatures on the Celsius scale can be positive or negative.
The Fahrenheit scale sets 32°F at the freezing point of water and 212°F at the boiling point of water. The Fahrenheit scale is primarily used in the United States.
The Kelvin scale is an absolute scale with 0 K representing absolute zero, the theoretical lowest temperature where molecular motion stops. The Kelvin and Celsius scales are offset by 273.15 degrees, so 0°C is equal to 273.15 K. The Kelvin scale does not use negative numbers.
Having standardized temperature scales allows us to precisely measure and communicate temperature for scientific, industrial, medical, and everyday applications. The choice of scale depends on the region and use case.
Applications of Temperature
Temperature measurement and control are crucial in many areas of everyday life and industry. Here are some key applications of temperature:
Cooking
The temperature of ovens and stovetops is carefully regulated to properly cook food. Different dishes require specific temperature ranges to reach the desired doneness and texture. Food safety also depends on maintaining proper temperatures.
HVAC

Heating, ventilation and air conditioning (HVAC) systems rely on thermostats to measure and adjust indoor temperatures for comfort and efficiency.
Weather Forecasting
Measurements of air, ground and water temperatures factor into weather forecasts around the globe. Temperature heavily influences weather patterns.
Medical Diagnosis
Doctors measure body temperature during physical exams to check for fever and other conditions. Precise thermometers help diagnose issues.
Industrial Processes
Many industrial processes involve heating or cooling materials to exact temperatures. Temperature control is vital for manufacturing, chemical reactions, food processing, and more.
Chemical Reactions
The rate of chemical reactions depends on the temperature. Chemists carefully regulate temperature to optimize reactions.
Space Exploration
Spacecraft and satellites contain thermometers to monitor temperature fluctuations in space. This helps identify issues and protects sensitive equipment.
Energy Transfer and Temperature
Temperature is fundamentally related to energy transfer between objects or systems. According to the second law of thermodynamics, heat will naturally flow from an object at a higher temperature to an object at a lower temperature until they reach thermal equilibrium.
For example, when a hot cup of coffee sits on a table, the coffee will transfer heat to the cooler table and surroundings, causing the coffee to decrease in temperature while the table gains heat and increases in temperature slightly. This heat transfer continues until the coffee and table reach the same temperature and there is no longer any net heat flow between them.
This spontaneous heat flow from hot to cold is why temperature differences are so critical for many energy applications. Heat engines like car engines rely on high and low temperature reservoirs to create power. Air conditioners move heat from indoors to outdoors to provide cooling. And heat pumps can transfer heat in either direction to maintain comfortable indoor temperatures.
Understanding that temperature differences drive energy transfer gives us control over thermal systems and allows us to harness temperature gradients for useful work and heating/cooling applications.
Temperature in Physics Laws
Temperature is an important factor in several fundamental physics laws that describe the behavior of energy and matter. Two of the most notable examples are the ideal gas law and the laws of thermodynamics.
The ideal gas law states that the pressure, volume, and temperature of a gas are directly proportional, assuming the amount of gas remains constant. Specifically, PV = nRT, where P is pressure, V is volume, n is the number of moles of gas particles, R is the ideal gas constant, and T is temperature in Kelvin. This law demonstrates that temperature is tied to the kinetic energy of gas particles and relates to their pressure and volume.
The laws of thermodynamics also incorporate temperature as a key variable. The zeroth law of thermodynamics involves the transitive nature of temperature equilibrium between multiple bodies. The first law describes how internal energy, heat, and work are interrelated. The second law defines entropy and requires that total entropy can never decrease over time for an isolated system. The third law states that absolute zero is unattainable. Overall, these laws describe how heat and temperature are linked to fundamental concepts like energy, entropy, and work.
Conclusion
In summary, temperature is a measure of the average kinetic energy of molecules in a substance. As molecules move faster and have more kinetic energy, the temperature increases. While temperature is related to energy and measures the energy present as molecular motion, temperature itself is not energy. Rather, temperature provides information about the energy contained within a system.
While heat and thermal energy refer to the total energy of molecular motion in a substance, temperature specifically measures the average of that kinetic energy. Temperature is an intensive property, meaning it does not depend on the amount of matter present. This allows temperature to be used to compare substances regardless of sample size.
Understanding the relationship between temperature and energy is key in many areas of science and engineering. Though temperature does not directly quantify energy, the two are intrinsically linked. Measuring temperature provides insight into the kinetic energy and thermal properties of materials and systems.