Is Thermal Energy Kinetic Energy?
Define Thermal Energy
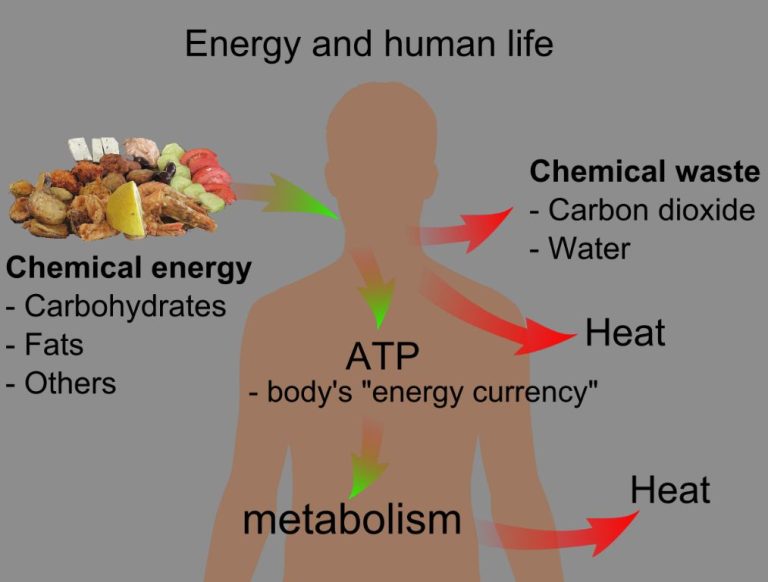
Thermal energy refers to the total kinetic energy of all the molecules within a substance. It is directly related to the temperature of the substance. Temperature is a measure of the average kinetic energy of the molecules or atoms in a substance. The higher the temperature, the greater the thermal energy since the molecules and atoms are moving faster and colliding more forcefully with each other.
Thermal energy comes from the motions and vibrations of the atoms and molecules in matter. The more kinetic energy the atoms or molecules possess, the higher the thermal energy. Thermal energy flows from substances at higher temperatures to substances at lower temperatures until equilibrium is reached. This transfer of thermal energy is called heat.
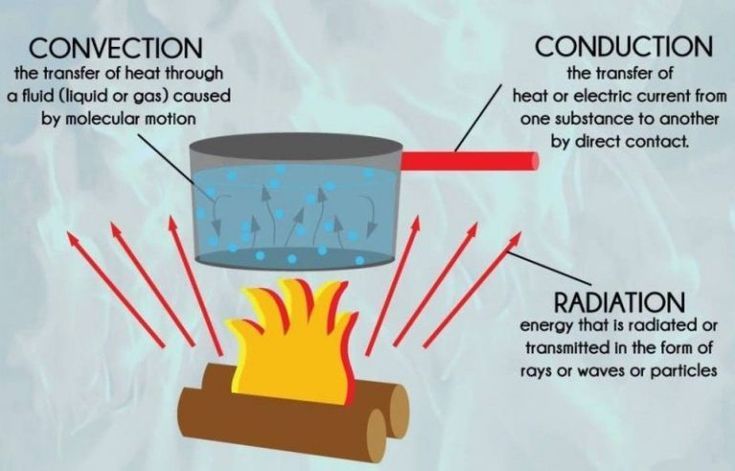
Thermal energy is present in all matter. Even substances that feel cold have thermal energy because their atoms and molecules are in constant motion. Thermal energy can be transferred between substances through processes like conduction, convection, and radiation.
Define Kinetic Energy
Kinetic energy is the energy of motion. An object that has motion – whether it is vertical or horizontal motion – has kinetic energy. The amount of kinetic energy depends on the mass and velocity of the object. The greater the mass and velocity, the greater the amount of kinetic energy. For example, a train has a huge mass and moves at high velocities, so it contains a large amount of kinetic energy.
Some key points about kinetic energy:
- Any object in motion has kinetic energy.
- Kinetic energy depends on an object’s mass and velocity.
- The more mass an object has and the faster it moves, the greater its kinetic energy.
- Kinetic energy is directly proportional to mass and velocity squared.
- Kinetic energy is a form of mechanical energy.
- Kinetic energy is converted into other forms of energy when an object slows down or stops moving.
In summary, kinetic energy is the energy possessed by objects due to their motion. The amount of kinetic energy depends on the object’s mass and velocity.
Relationship Between Temperature and Kinetic Energy
There is a direct link between temperature and the kinetic energy of molecules. Temperature is a measure of the average kinetic energy of molecules. As molecules gain kinetic energy, they move faster and collide more forcefully with each other and their container. This increased molecular motion corresponds to an increase in temperature.
When a substance is heated, energy is transferred to the molecules, increasing their kinetic energy. The molecules vibrate faster and move more rapidly. As the average kinetic energy increases, the temperature rises. Conversely, removing heat lowers the average kinetic energy and decreases temperature.
In summary, temperature and kinetic energy are directly proportional. Higher temperatures mean the molecules have greater average kinetic energy. Lower temperatures mean the molecules have lower average kinetic energy.
Thermal Energy Sources
Thermal energy comes from the kinetic energy of atoms and molecules. Atoms and molecules are constantly in motion, vibrating and colliding with each other. The faster they move, the more kinetic energy they have. This kinetic energy is directly related to an object’s temperature.
Heat is transferred when atoms and molecules with high kinetic energy collide with atoms and molecules with lower kinetic energy. The high kinetic energy is transferred to the lower kinetic energy atoms, increasing their motion. This transfer of kinetic energy from high to low energy atoms is the source of an object’s thermal energy.
Some common sources that provide thermal energy include:
- Friction – Kinetic energy is converted to thermal energy when two surfaces rub against each other.
- Chemical reactions – Exothermic chemical reactions like combustion transfer some of the kinetic energy from the reaction into thermal energy.
- Electrical current – As electrons move through a conductor, they collide with atoms and transfer kinetic energy as heat.
- Nuclear reactions – Fission and fusion involve changes in atomic nuclei that release large amounts of kinetic energy as heat.
- Sunlight – The sun’s rays transfer kinetic energy to objects on Earth, heating them up.
In all these examples, thermal energy ultimately comes from the kinetic energy at the atomic and molecular level. Thermal energy sources rely on transferring kinetic energy from one body to another.
Is Thermal Energy Kinetic Energy?
Thermal energy, also known as heat energy, is the energy transferred between objects or systems due to their temperature difference. Kinetic energy is the energy possessed by an object in motion. While the two types of energy seem very different at the macro level, thermal energy is actually a manifestation of kinetic energy at the microscopic level.
Thermal energy comes from the kinetic energy of atoms and molecules in matter. Atoms and molecules are always in motion, vibrating and moving around even in stationary objects. The higher the temperature of matter, the faster the atoms and molecules vibrate and move. This microscopic kinetic motion gets transferred through collisions between neighboring particles. As the atoms and molecules bump into each other, their kinetic energy is transferred throughout the material.
So in summary, thermal energy comes from the kinetic energy of atoms and molecules. The greater the atomic/molecular motion in a material, the more thermal energy it possesses. Heating matter increases the speed of the particles, generating greater kinetic energy that is observed as a temperature rise. Cooling matter slows down the particles, decreasing their kinetic energy and lowering the temperature. Thermal energy and temperature are macroscopic manifestations of the microscopic kinetic energy within matter.
Examples of Thermal Energy
Thermal energy, or heat, can take many different forms. Some common examples of thermal energy include:
Heat – Heat is often used interchangeably with thermal energy and refers to the total kinetic energy of molecules within an object. As an object gets hotter, its molecules vibrate and move faster, increasing the thermal energy.
Internal Energy – This refers to the total kinetic and potential energy of all the molecules within a thermodynamic system. Internal energy is directly related to an object’s temperature.
Other examples of thermal energy include the warmth provided by the sun, friction heat generated when rubbing your hands together, and geothermal energy from the Earth’s core.
Any process or activity that involves motion and vibrating molecules can produce thermal energy. Even living organisms generate thermal energy from cellular processes and metabolism.
Using Thermal Energy
Thermal energy has many practical uses and applications. The most common use of thermal energy is for heating and cooling. We rely on the transfer of thermal energy to heat our homes, cook our food, and regulate the temperature of objects. Thermal energy flows from warmer objects to cooler objects through conduction, convection, and radiation.
Thermal energy can also be harnessed to perform useful work through heat engines. Heat engines convert thermal energy into mechanical work. Some examples of heat engines are:
- Internal combustion engines – Gasoline and diesel engines use the controlled burning of fuel to create pressure that moves pistons.
- Steam engines – Boiling water produces steam that pushes a piston or turbine.
- Gas turbines – Hot gases spin a turbine to generate electricity.
In these heat engines, there is a heat source (fuel combustion, steam boiler) that provides thermal energy. This thermal energy is transferred to a working fluid, expanding it and causing motion. The motion is then harnessed through pistons, turbines or other devices to perform mechanical work. This allows us to power vehicles, generate electricity, and more using thermal energy.
Measuring Thermal Energy
There are two main ways to measure thermal energy:
Temperature
Temperature is a measure of the average kinetic energy of particles in a substance. It is measured using a thermometer. Higher temperatures mean the particles have more kinetic energy on average.
Calorimetry
Calorimetry allows us to measure the amount of heat energy transferred between substances. This involves putting a sample in a calorimeter – an insulated container designed to minimize energy transfer between the sample and surroundings. The temperature change of the sample is used along with its mass and heat capacity to calculate the amount of thermal energy.
Calorimetry experiments provide quantitative data on the thermal energy involved in physical and chemical changes.
Thermal Energy vs Temperature
Thermal energy and temperature are related, but distinct concepts. Thermal energy refers to the total kinetic energy of all the molecules in an object. Temperature measures the average kinetic energy of the molecules.
As an object gains thermal energy, the molecules move faster and their average kinetic energy increases, resulting in a higher temperature. However, two objects can have the same temperature but different thermal energy, if they have a different number of molecules.
For example, a bathtub of hot water and a cup of hot water may be the same temperature, say 50°C, but the bathtub has much greater thermal energy because it contains many more water molecules. The average kinetic energy per molecule is the same, but the total kinetic energy contained in the bathtub is much greater.
In summary, temperature provides a measure of the average molecular kinetic energy, whereas thermal energy refers to the total kinetic energy contained in an object. While related, they are distinct properties of matter.
Conclusion
To summarize, while thermal energy and kinetic energy are related, they are ultimately distinct forms of energy. Thermal energy refers to the total internal energy of a system arising from the motion of its atoms and molecules. An increase in thermal energy is associated with an increase in the temperature of a system. Kinetic energy, on the other hand, refers specifically to the energy an object possesses due to its motion. The atoms and molecules that make up a system have kinetic energy due to their motion. An increase in the average kinetic energy of the particles in a system corresponds to an increase in the temperature of that system. While an increase in thermal energy is often accompanied by an increase in kinetic energy at the particle level, they remain separate forms of energy. Thermal energy is internal energy related to the temperature of a system, while kinetic energy is energy of motion.