What Is Associated With Thermal Energy?
What is Thermal Energy?
Thermal energy refers to the internal energy present in a system due to the motion of molecules and atoms. It is directly related to the temperature of matter; the higher the temperature, the greater the thermal energy. Thermal energy is essentially the energy associated with heat. While heat and thermal energy are often used interchangeably, they have distinct scientific meanings.
Heat is the transfer of thermal energy between systems, while thermal energy refers specifically to the total kinetic energy of molecules within a system. As an object is heated up, its molecules gain kinetic energy and vibrate and move faster. This increase in molecular motion corresponds to an increase in thermal energy. So heat describes the flow of thermal energy from high temperature to low temperature, while thermal energy refers to the internal energy contained in a system due to molecular motion.
Thermal energy is generally measured in joules or calories. It can be converted into other forms of energy like mechanical energy. Understanding thermal energy is important across many fields from engineering to biology to cooking, as regulating and utilizing thermal energy has countless applications in our lives.
Forms of Thermal Energy
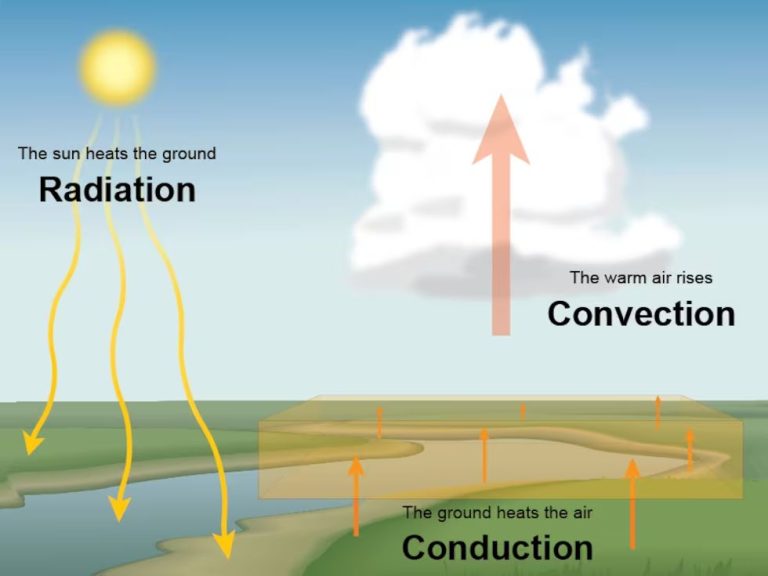
Thermal energy is present in different forms depending on how heat is transferred. The three main forms of thermal energy are conduction, convection, and radiation.
Conduction is the transfer of heat through direct contact. For example, a spoon left in a hot cup of coffee will become hot as the heat is conducted through the metal. The more conductive a material, the more easily heat will transfer through it. Metals like copper are good conductors.
Convection is the transfer of heat through fluids or gases. As a fluid or gas is heated, it expands, becomes less dense, and rises. Cooler, denser fluid or gas will then take its place. This movement distributes heat through convection currents. Examples include boiling water and the way heat circulates in a room.
Radiation is the transfer of heat in the form of electromagnetic waves. No direct contact is needed. The heat we feel from the sun is an example of radiant heat. Other examples include heat from a fireplace warming a room and an electric heater heating a space.
Measuring Thermal Energy
Thermal energy is most commonly measured using the following units:
- Joules – The joule is the SI unit for energy and work. It is defined as the work done by a force of one newton moving an object one meter. Joules are commonly used to measure thermal energy in scientific contexts.
- Calories – The calorie was originally defined as the amount of energy needed to raise one gram of water by one degree Celsius. In nutrition, the Calorie (upper case C) refers to the large calorie or kilocalorie, which is equal to 1000 calories. Calories are commonly used to measure thermal energy in food.
- British Thermal Units (BTUs) – The BTU is a traditional unit of energy equal to about 1055 joules. It is approximately the amount of energy needed to heat one pound of water by one degree Fahrenheit. BTUs are commonly used to measure thermal energy in heating applications.
These units allow us to precisely quantify thermal energy for calculations and comparisons. Devices like thermometers, calorimeters, and flow meters help measure temperature changes and energy transfers that indicate the amount of thermal energy present or moved.
Thermal Energy Transfer
Thermal energy refers to the total kinetic energy of molecules within an object. This energy can be transferred between objects through conduction, convection, and radiation:
Conduction is the transfer of thermal energy between objects in direct physical contact. Heat flows from the object with higher temperature to the object with lower temperature until they reach thermal equilibrium. The rate of conductive heat transfer depends on the temperature difference and the thermal conductivity of the materials. Metals like copper and aluminum are good conductors.
Convection is the transfer of thermal energy by the movement of heated fluid. Fluids expand when heated, becoming less dense, and rise while cooler, denser fluid sinks. This causes circulation that transfers heat. Convection occurs in liquids like water and gases like air. It can be natural (from temperature differences) or forced (from fans or pumps).
Radiation is the transfer of thermal energy by electromagnetic waves directly between objects, even through empty space. No direct contact is required. The rate of radiative heat transfer depends on the temperature of the objects and their emissivity. Dark and rough surfaces are good emitters and absorbers of radiant energy.
Understanding thermal energy transfer mechanisms allows effective use and control of heat flow for heating, cooling, insulation, and energy efficiency.
Effects of Thermal Energy
Thermal energy can have significant effects on matter. Two of the most notable effects are thermal expansion/contraction and phase changes.
Thermal expansion occurs when matter increases in volume as it gains thermal energy and heats up. The molecules gain kinetic energy and move farther apart, taking up more space. This causes the material to expand in size. Metals are especially prone to noticeable expansion. When cooled down, the reverse effect of thermal contraction happens as matter shrinks in volume. This shrinking effect can be useful for fitting parts together tightly.
Phase changes are the transition of matter between solid, liquid, and gas phases. These changes occur because of the kinetic energy of molecules. As thermal energy increases and molecules gain kinetic energy, their attractions weaken and they move freer, transitioning from solid to liquid to gas. Adding or removing thermal energy can induce these phase changes. For example, applying heat can melt a solid into a liquid and vaporize a liquid into a gas. Removing heat through cooling can cause a gas to condense into a liquid and freeze into a solid.
The effects of thermal expansion/contraction and phase changes demonstrate that thermal energy can alter the properties and state of matter in significant ways. Understanding these effects has allowed scientists to develop many useful applications that rely on the ability of thermal energy to transform materials.
Thermal Energy Applications
Thermal energy has many practical applications in our everyday lives. Some of the most common ways we utilize thermal energy include:
Engines
Most engines rely on thermal energy to operate. In car engines, for example, fuel is burned to release thermal energy which creates pressure that moves the pistons. This thermal energy is converted into mechanical energy that propels the vehicle. Engines in everything from household generators to rockets function similarly, using thermal energy as an input to create useful work.
Heating and Cooling
Heating and cooling systems also leverage thermal energy. Furnaces, boilers, and heaters use thermal energy from burning fuel to warm air or water. Air conditioners and refrigerators move thermal energy from one place to another, cooling an interior space. Controlling ambient temperatures for comfort is one of the most widespread uses of thermal energy in buildings and vehicles.
Cooking
The cooking process fundamentally involves transferring thermal energy into food. Appliances like ovens, stoves, and grills use thermal energy sources like electricity, gas, or charcoal to heat food. This application of thermal energy transforms ingredients by softening, caramelizing, maillardizing, and more. Thermal energy allows us to make food safer, tastier, and more digestible.
Thermal Energy Storage
Thermal energy storage allows excess thermal energy to be captured and stored for later use. There are several ways to store thermal energy, including batteries, phase change materials, and thermal mass.
Batteries convert chemical energy into electrical energy and vice versa. Rechargeable batteries like lithium-ion can store energy for repeated use. Thermal management systems help regulate battery temperatures for optimal efficiency and prevent overheating.
Phase change materials (PCMs) use chemical bonds to absorb and release heat. The most common PCMs are organic compounds like paraffin wax. As PCMs change phase from solid to liquid, they absorb thermal energy. This energy can be released later during solidification. PCMs are used in buildings to reduce indoor temperature fluctuations.
Thermal mass utilizes dense materials like stone, concrete, or water to absorb and store heat. Massive objects resist temperature change and can slowly release absorbed energy over time. In passive solar design, thermal mass buffers indoor spaces against external temperature swings.
Thermal Energy and Life
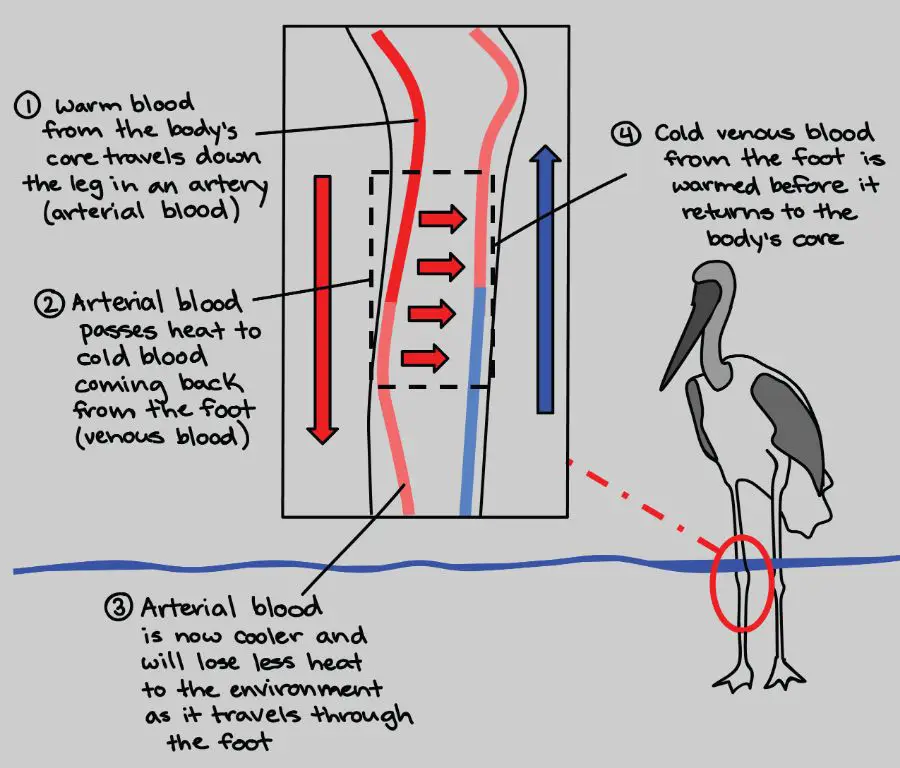
Thermal energy plays a vital role in the lives of plants and animals. Animals, including humans, utilize thermal energy to regulate body temperature. Endothermic animals like mammals and birds maintain a constant internal body temperature despite varying external temperatures. They do this by generating heat metabolically and through mechanisms like shivering and sweating. Ectotherms like reptiles and amphibians rely on external thermal energy sources like the sun to regulate their body temperature. Plants also depend on thermal energy from sunlight for photosynthesis and other metabolic processes. The ambient temperature affects the rates of these chemical reactions and growth cycles in plants. Thermal energy impacts behaviors in animals like hibernation, aestivation and migration which are adaptations to deal with temperature extremes. Thermal energy from forest fires also plays a role in the lifecycle of certain plant species by triggering seed germination. Overall, the utilization and regulation of thermal energy is essential to the survival and propagation of flora and fauna.
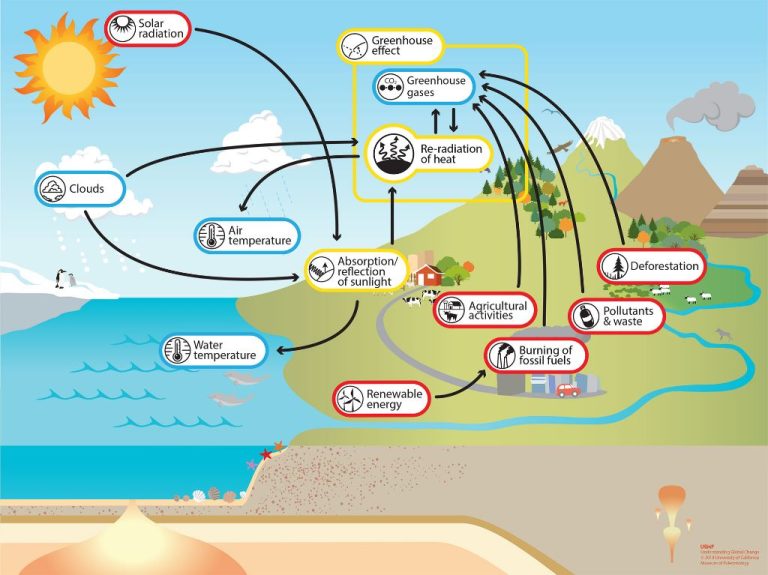
Thermal Energy and Climate
Thermal energy plays a significant role in Earth’s climate. The greenhouse effect is a natural process where certain gases in the atmosphere, called greenhouse gases, trap some of the Sun’s heat. This raises Earth’s average surface temperature to about 15°C, compared to -18°C if the greenhouse effect did not exist. The most abundant greenhouse gases are water vapor, carbon dioxide, methane, nitrous oxide, and ozone.
Human activities like burning fossil fuels have increased greenhouse gas levels in the atmosphere, amplifying the natural greenhouse effect and causing global warming. Global warming leads to rising sea levels, melting glaciers, more extreme weather, ecosystem changes, and more. If greenhouse gas emissions continue increasing at the current rate, Earth’s average temperature could rise by 2-6°C by 2100. This would severely disrupt weather patterns, ecosystems, and food and water supplies around the world.
Reducing greenhouse gas emissions is crucial to mitigate climate change. This involves transitioning from fossil fuels to renewable energy sources like solar, wind, hydroelectric, geothermal and biofuels. Improving energy efficiency in buildings, transportation and industry can also curb emissions. Additionally, protecting and expanding forests is important, as trees absorb and store carbon dioxide.
Fun Facts About Thermal Energy
Here are some interesting and fun facts about thermal energy:
-
The hottest temperature ever recorded on Earth was 136°F in Libya in 1922.
-
The coldest temperature ever directly recorded on Earth was -129°F in Antarctica in 1983.
-
The sun produces so much thermal energy that it could power the entire world for a full year with just one second of its energy production.
-
According to the laws of thermodynamics, thermal energy can never be fully destroyed or created – it can only be transferred from one location to another.
-
Thermal energy transfer is used in many common technologies and appliances, from refrigerators to air conditioners to heat pumps.
-
Many animals and plants have evolved special adaptations that help them survive in extreme heat or cold.
-
The amount of thermal energy contained within the Earth’s core is estimated to be able to continuously produce heat for billions of years due to radioactive decay.
Thermal energy may seem like a simple concept, but it has many fascinating applications and implications in our everyday lives and throughout the natural world.