Is Temperature A Thermal Or Kinetic Energy?
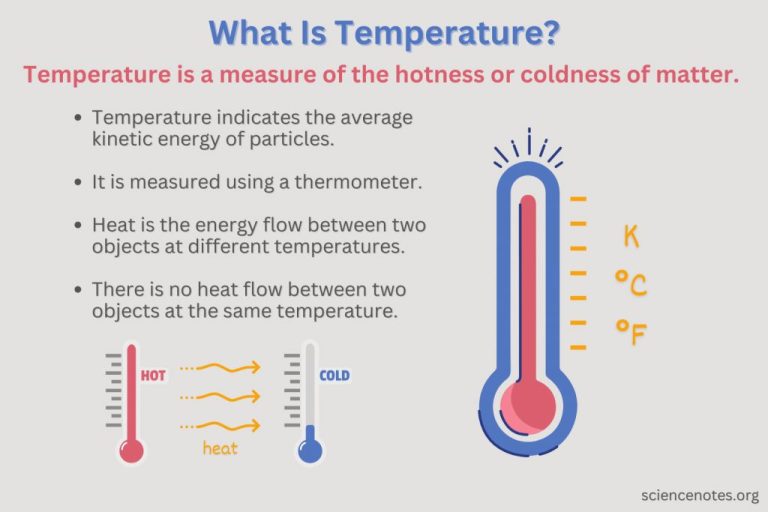
What is Temperature?
Temperature is a measure of the average kinetic energy of molecules and atoms. It refers to how hot or cold an object is based on the internal energy and motion of its molecules.
Temperature is measured using thermometers and temperature scales. The most common scales are Celsius, Fahrenheit, and Kelvin. On the Celsius scale, water freezes at 0°C and boils at 100°C. On the Fahrenheit scale, water freezes at 32°F and boils at 212°F. The Kelvin scale is an absolute scale where 0 Kelvin is absolute zero.
Temperature is an intrinsic property of matter that determines the direction of heat flow between two objects. Heat will flow from higher temperature objects to lower temperature objects until equilibrium is reached. This relationship allows us to measure temperature with various devices like thermometers.
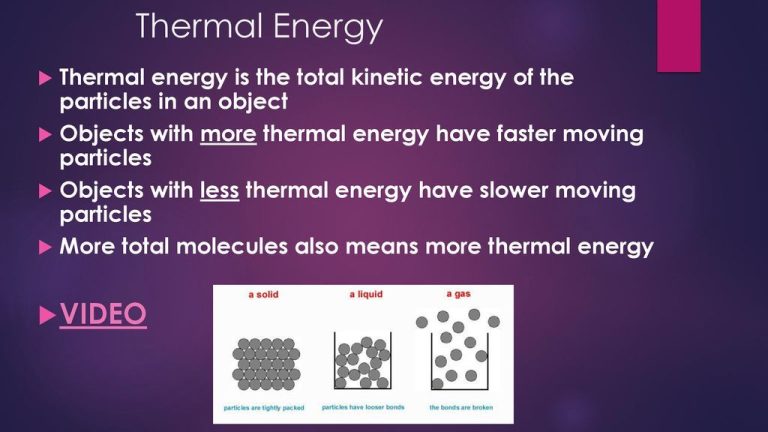
Thermal vs Kinetic Energy
Thermal energy is the total potential and kinetic energy associated with the random motion of the molecules and atoms of a substance. It arises from the motions of the microscopic constituents of matter. The more motion the molecules and atoms have, the higher the thermal energy.
Kinetic energy is the energy possessed by an object in motion. It depends on the mass and velocity of the object and is calculated as 1/2mv2, where m is mass and v is velocity. Kinetic energy is a form of mechanical energy associated with the gross motion and speed of an entire object or system.
The key differences between thermal and kinetic energy are:
- Thermal energy is associated with the random molecular motion of a substance, while kinetic energy refers to the bulk motion of an entire object.
- Thermal energy depends on the temperature, while kinetic energy depends on the mass and velocity.
- Thermal energy is present even when objects are at rest, while kinetic energy requires overall motion.
- Thermal energy is a microscopic effect, while kinetic energy is a macroscopic property.
Examples of thermal energy include the internal energy of a gas that arises from collisions between molecules. Kinetic energy examples include a rolling ball, flowing water, or a person running.
Relationship Between Temperature and Energy
There is an important relationship between temperature and energy at the molecular level. Temperature is a measure of the average thermal energy or heat in a substance. It indicates how much internal energy the molecules within a substance possess. The higher the temperature, the greater the average kinetic energy of the molecules.
As molecules gain thermal energy, they vibrate and move faster, colliding more frequently with each other and their container. Their increased kinetic energy is detected as a rise in temperature. When heat is lost, molecular motion decreases, reducing kinetic energy and lowering temperature.
Therefore, temperature provides a direct measure of the mean kinetic energy of molecular motion in a substance. Thermal energy causes molecules to vibrate, rotate, and translate, while temperature indicates the average kinetic energy involved in this molecular motion. They are intrinsically linked at the particulate level.
Temperature and Molecular Motion
Temperature is a measure of the average molecular kinetic energy or molecular motion in a substance. As kinetic energy increases, molecular motion increases, and temperature rises. Molecular motion is related to thermal energy. The greater the thermal energy or heat in a system, the faster the molecules vibrate and move.
Molecules are always in motion, even in the solid phase of matter. As additional thermal energy is added to a system, molecular vibration and motion increase. The increase in molecular kinetic energy is perceived macroscopically as a temperature rise. When thermal energy is removed, molecular motion decreases, and temperature drops.
Therefore, temperature is ultimately a reflection of the kinetic energy of molecules and the intensity of their motion and vibration within a system. This makes temperature a kinetic phenomenon and direct indicator of molecular motion and kinetic energy.
Measuring Temperature
There are various devices used to measure temperature, the most common being thermometers. Thermometers work by correlating a property of a sensor material that changes with temperature to the temperature scale. For example, mercury or alcohol thermometers rely on the expansion and contraction of a liquid with temperature. As the liquid heats up and expands, it rises within a sealed glass tube calibrated with temperature markings. Other types of thermometers include:
Thermocouples – Consist of two dissimilar metal wires joined at one end. The junction produces a small voltage dependent on temperature. Thermocouples are inexpensive, durable, have a fast response time, and large temperature range.
Resistance temperature detectors (RTDs) – Use electrical resistance and its known dependence on temperature in pure metals like platinum, copper or nickel. More accurate than thermocouples but slower response time.
Thermistors – Semiconductor-based sensors that exhibit a large change in electrical resistance with small changes in temperature. Used in digital thermometers, thermostats, and temperature measurement systems.
Infrared thermometers – Measure infrared energy radiated from an object’s surface to determine its temperature without contact. Used in industrial settings and medical applications.
Other advanced devices for measuring temperature include pyrometers and thermographic cameras. Each type of temperature sensor has advantages and limitations depending on the application requirements for range, accuracy, response time and robustness.
Units of Temperature
Temperature is measured in various scales and units. Some of the most common are:
Celsius
The Celsius scale, also known as centigrade, is commonly used in science. On this scale, 0°C is the freezing point of water and 100°C is the boiling point of water. Each degree Celsius represents 1/100th of the difference between the freezing and boiling points of water.
Fahrenheit
The Fahrenheit scale is commonly used in the United States. On this scale, 32°F is the freezing point of water and 212°F is the boiling point of water. The Fahrenheit scale uses 180 degrees between the freezing and boiling points of water.
Kelvin
The Kelvin scale is the standard unit of temperature used in science. On this scale, 0 K represents absolute zero, the theoretical lowest temperature where molecular motion stops. Water freezes at 273.15 K and boils at 373.15 K. Each Kelvin degree represents the same temperature difference as 1 Celsius degree.
Temperatures can be converted between different units using conversion formulas:
- Celsius to Fahrenheit: F = C x 1.8 + 32
- Fahrenheit to Celsius: C = (F – 32) / 1.8
- Celsius to Kelvin: K = C + 273.15
- Kelvin to Celsius: C = K – 273.15
Understanding the different temperature scales and how to convert between them is important for fields like science, engineering, and meteorology where precise temperature measurements are critical.
Heat vs Temperature
There is an important distinction between heat and temperature that is worth understanding. While the two are related, they refer to different properties.
Heat is a form of energy transfer between objects or systems due to a temperature difference. Heat always flows from higher temperature to lower temperature. For example, when you boil water on a stove, the stove elements get hotter than the water and transfer heat into the water, increasing its temperature.
Temperature, on the other hand, is a measure of the average kinetic energy of molecular motion in a substance. Higher temperatures mean the molecules are moving faster on average. As heat is added to a substance, molecular motion increases as the molecules gain kinetic energy. This increase in molecular kinetic energy is perceived as a rise in temperature.
So in summary, heat refers to energy being transferred between objects, while temperature indicates the internal energy state of a system or object based on molecular motion. Understanding this distinction is important when studying thermodynamics.
Examples and Applications
Temperature is a measure of molecular motion or kinetic energy. We can observe examples of this relationship between temperature and kinetic energy in everyday phenomena:
Boiling Water
As water is heated, the water molecules gain kinetic energy and begin moving faster and more erratically. Once the kinetic energy reaches a certain level, the water reaches its boiling point (212°F at sea level), at which point liquid water transitions to water vapor (steam). The boiling point is a constant indicator of the kinetic energy state of the water molecules.
Melting Ice
When ice is subjected to heat, it begins absorbing thermal energy. As the water molecules in ice gain kinetic energy, the ice begins melting into liquid water once a certain temperature (32°F at sea level) is reached. This melting point indicates the molecules have reached sufficient kinetic energy to break out of the rigid crystal lattice of ice.
Thermometers
Thermometers take advantage of the relationship between molecular kinetic energy and temperature. As molecules collide with the thermometer (e.g. mercury in a glass thermometer), they transfer kinetic energy in the form of heat. This causes the temperature indicator (mercury level) to rise proportionally with the kinetic energy and temperature.
Summary
We learned that temperature is related to thermal energy while kinetic energy refers to energy from motion. Temperature measures how hot or cold something is and is demonstrated by the kinetic energy or molecular motion in a material. Both thermal energy and temperature involve molecular motion but one measures the total energy while the other measures intensity. We also covered how to measure temperature in different units like Celsius, Fahrenheit, and Kelvin. The key difference between heat and temperature is that heat is energy transferred between objects while temperature is the internal thermal energy of a system. Temperature is an important concept with many applications in science, engineering, weather, and everyday life.
References
[1] Author, A. Book Title. Publisher, Year.
[2] B. Author, “Article Title,” Journal Title, vol. #, no. #, pp. #-#, Year.
[3] C. Author, “Webpage Title,” Website, Date Accessed. [Online]. Available: URL.
[4] D. Author, “Page Title,” Website, Date Published/Updated [Date Accessed]. Available: URL.
[5] E. Author, Title of Work, Medium/Site Name, Year Published, Date Accessed. Available: URL.