What Is Thermal Energy That Is Transferred Called?
What is Thermal Energy?
Thermal energy refers to the total kinetic energy and potential energy of atoms and molecules within an object or system. It is often referred to as heat energy. Thermal energy arises from the random motions of atoms and molecules as they vibrate and move. The higher the temperature of a system or object, the greater the thermal energy contained within it.
Temperature is a measure of the average kinetic energy of particles in a substance. Thermal energy flows from higher to lower temperatures. All matter consists of atoms and molecules that are in constant motion. This kinetic energy of microscopic motion is thermal energy. Even objects or substances that feel cold have thermal energy – their atoms are just moving slower on average.
Thermal energy is important because it can be transferred from one object or system to another in three main ways – conduction, convection, and radiation. This transfer of thermal energy is key to many everyday phenomena and technologies, as well as larger processes that shape the physical world.
Methods of Thermal Energy Transfer
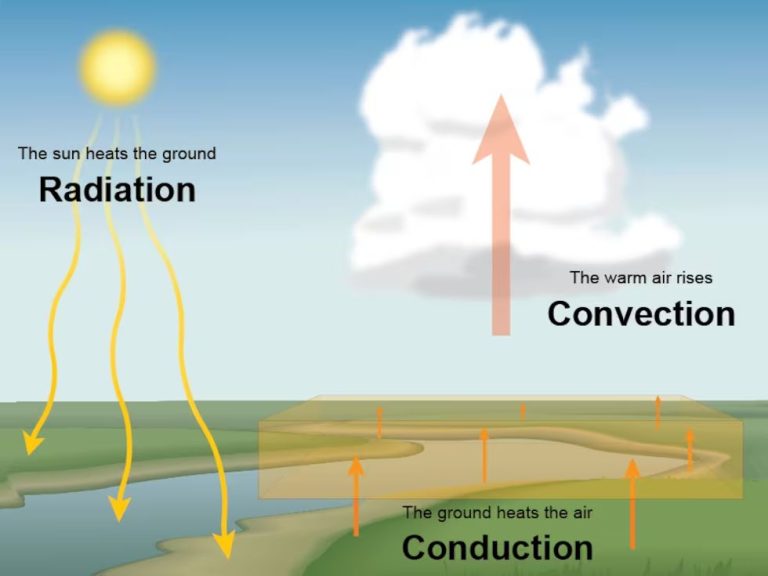
There are three main methods by which thermal energy, also known as heat, is transferred from one object or system to another: conduction, convection, and radiation.
Conduction
Conduction is the transfer of thermal energy between objects that are in direct physical contact with each other. It occurs when atoms and molecules interact, transferring kinetic energy from more energetic particles to less energetic ones. Metals are good conductors of thermal energy.
Convection
Convection is the transfer of thermal energy by the motion of fluids. It occurs when warmer areas of a liquid or gas rise to cooler areas, causing circulation currents. Convection takes place in liquids like water and air.
Radiation
Radiation is the transfer of thermal energy via electromagnetic waves directly between objects, without requiring direct contact. It can travel through vacuums. The thermal radiation emitted by hot objects like the sun can be absorbed by cooler objects.
Conduction
Conduction is the transfer of thermal energy that occurs through direct contact between objects. It involves the transfer of kinetic energy between molecules and electrons that are in direct physical contact. For example, when you place a metal spoon in a hot cup of soup, the metal spoon quickly becomes hot. This occurs because the spoon is in direct contact with the hot soup, allowing the faster moving soup molecules to collide with the slower moving spoon molecules, transferring kinetic energy directly between them.
Conduction can only occur between objects that are touching, and it tends to be most effective in solid materials, where molecules are more tightly packed. Metals in particular are very good conductors of thermal energy. The rate of conductive heat transfer depends on the temperature difference between the objects in contact and the properties of the materials themselves.
Convection
Convection is one type of thermal energy transfer that occurs through fluids like liquids and gases. When a fluid is heated, it expands, becomes less dense, and rises. As the heated fluid rises, cooler denser fluid sinks to take its place, creating circulation or convection currents. This transfer of heat energy by the movement of fluids is called convection.
A common example of convection is a pot of water boiling on a stove. The heated water at the bottom of the pot expands, becomes less dense, and rises to the top. Meanwhile, the cooler water sinks down to take its place. This circulation distributes the heat throughout the pot until all the water reaches the boiling point.
Radiation
Radiation refers to the transfer of thermal energy through electromagnetic waves, or photons, that are emitted by all objects with a temperature greater than absolute zero. Unlike conduction and convection, radiation does not require any medium for the transfer of heat and can even occur in a vacuum. For example, the sun transfers thermal energy to the Earth across empty space by radiation. The sun emits electromagnetic waves that travel through space and are absorbed by the Earth, heating its surface. All objects that have a temperature emit thermal radiation, with hotter objects emitting more intense radiation than cooler objects. Radiation does not involve any actual movement or flow of matter, only the propagation of electromagnetic waves.
Comparing Methods of Transfer
The three main methods of thermal energy transfer—conduction, convection, and radiation—each have their own unique characteristics regarding speed, distance, mediums, and use cases.
Conduction transfers heat through direct contact between materials and is most effective over short distances. It occurs fastest in solid mediums like metals but does not occur at all in air. Conduction is used in cooking with metal pots and pans as well as in electronics cooling systems.
Convection relies on the movement of fluids like air or water to transfer heat and can happen over longer distances than conduction. It is slower than conduction but faster than radiation. Convection occurs in water heating systems, the atmosphere, and ocean currents.
Radiation transfers heat remotely through electromagnetic waves and does not require contact between source and destination. It can transfer heat over long distances through vacuums or air. Radiation is the slowest method but essential for transferring solar energy to Earth. It is used in electric heaters, wood-burning stoves, and geothermal heating.
Overall, conduction is fastest over short distances in solids while radiation is slowest but capable of long distances through any medium. Convection provides a middle ground in speed and distance for liquid and gas mediums.
Thermal Energy Transfer in Real World Examples
Thermal energy is constantly being transferred in the world around us through conduction, convection, and radiation. Here are some common real world examples of thermal energy transfer:
Cooking: The heating element or burner of a stove conducts thermal energy to the metal pans placed on top of it. The metal pan then conducts the heat to the food inside the pan, cooking the food. Convection also occurs as hot air currents within the oven transfer heat to the food. Radiation plays a role too as the hot oven surfaces emit thermal radiation that is absorbed by the food.
Heating and cooling: In heating and cooling systems, convection is the primary means of thermal energy transfer. The heating or cooling unit blows hot or cold air through ducts which convectively transfers thermal energy throughout a building, car, etc. Radiators and baseboard heaters rely more on conduction, using hot water flowing through metal pipes to conduct heat.
Outer space: In the vacuum of space where matter is scarce, thermal energy is primarily transferred through radiation. For example, the sun’s core produces immense heat which is radiated outward through space to warm planets like Earth. On Earth’s surface, solar radiation is absorbed and re-emitted as infrared radiation, which is partly trapped in the atmosphere to create the greenhouse effect.
Importance and Applications
Thermal energy transfer plays a critical role in many areas of science, technology, and nature. Understanding how heat moves allows us to develop new technologies, predict weather patterns, and gain insights into Earth’s climate system.
In industry, knowledge of heat transfer is vital for improving efficiency in power generation, manufacturing, and chemical processing. Engineers apply principles of conduction, convection, and radiation to optimize heat flow in the design of motors, turbines, heat exchangers, boilers, furnaces, and other systems. Careful thermal management is crucial for maintaining proper operating temperatures and preventing overheating in computers, vehicles, and electronic devices.
The transfer of thermal energy drives convection in Earth’s atmosphere and oceans, playing a major part in ocean currents, wind patterns, and the global climate. It also governs everyday weather – from the formation of clouds and rainfall, to severe storms and lightning. Understanding atmospheric heat transfer is essential for meteorologists and climatologists.
In nature, animals rely on thermal regulation strategies like sweating, panting, and adjusting blood flow to maintain stable body temperatures. Plants optimize heat absorption for photosynthesis and transpiration. Thermal energy flows also impact ecosystems and natural environments, from deserts to coral reefs.
The three primary methods of heat transfer – conduction, convection, and radiation – are fundamental to the working of the natural world. Advances in technology and industry, meteorology, and many other fields depend on our scientific understanding of how thermal energy moves between objects and locations.
Interesting Facts About Thermal Energy Transfer
Here are some fascinating tidbits about thermal energy transfer that you may not know:
Hot air rises, cold air sinks. This is due to differences in air density. As air is heated, it expands and becomes less dense, so it will rise above denser, cooler air. This principle is key to thermal energy transfer through convection.
Radiant heating warms people directly. Unlike convection heating which relies on circulating air, radiant heating from sources like fireplaces and radiators warms up people and objects directly through infrared radiation.
Animals use conduction to stay warm. When kittens snuggle up close to their mother, they are conducting body heat between each other. Penguin huddles also conserve heat through conduction.
Bees use swarming to stay warm. Honeybees will form a tight cluster around the queen bee, buzzing their wing muscles to generate heat through vibration. The cluster can maintain temperatures of around 92°F even when outside temperatures drop below freezing.
Dark colors absorb heat better. Darker object colors like black absorb light and radiation better than lighter colors like white, causing them to heat up more rapidly.
Silver is the most conductive metal. Silver is the most thermally conductive metal, which is why it is often used for heat sinks and heat spreaders to dissipate heat.
Summary
In summary, thermal energy refers to the internal energy of an object or system due to the motion and interactions of its atoms and molecules. Thermal energy can be transferred from one object or system to another in three main ways:
Conduction is the transfer of thermal energy through direct contact between materials. It occurs when atoms or molecules with a lot of energy collide with and transfer energy to adjacent atoms and molecules with less energy. Metals are good conducting materials.
Convection is the transfer of thermal energy via the movement of heated liquids and gases. As the liquid or gas warms, it becomes less dense, rises, and carries thermal energy away from the source. This creates circulation currents that transfer heat through the entire liquid or gas.
Radiation is the transfer of thermal energy by electromagnetic waves. It does not rely on direct contact between materials or fluid circulation. Instead, thermal radiation is emitted by all objects and transmitted through space until absorbed by another object. The amount of radiation increases with temperature.
Understanding the three mechanisms of thermal energy transfer – conduction, convection, and radiation – is key to many engineering and science applications. These processes apply in everything from heat management in electronics to weather patterns in the atmosphere.