What Is Heat Energy Or Temperature?
What is Heat Energy?
Heat energy, or thermal energy, is the kinetic energy of atoms and molecules. It refers to the total energy of microscopic motions of particles in a substance. Heat is transferred between objects or systems when they have different temperatures.
On an atomic level, heat causes atoms and molecules to vibrate faster and spread apart. The more heat that is added, the faster the atoms and molecules move. This increases their kinetic energy, or energy of motion. The transfers of energy that occur due to a temperature difference between two objects or systems is called heat transfer.
For example, when a pot of water is heated on the stove, the heat from the stove burner causes the water molecules to move faster and spread farther apart. As the water absorbs this heat energy, its temperature rises. Heat energy can be transferred in three main ways – conduction, convection, and radiation.
What is Temperature?
Temperature is a measure of the average kinetic energy of particles in matter. It refers to how hot or cold an object is based on the motion of its atoms and molecules. As an object gains heat energy, its molecules and atoms vibrate and move faster, leading to an increase in temperature. Different temperature scales are used to measure this intensity of molecular motion.
On the molecular level, temperature is related to the kinetic energy of particles. Higher temperatures mean the particles have more energy and are moving faster on average. Lower temperatures correspond to slower molecular motion. Temperature measures how much hotness is present relative to a reference point. It does not directly quantify the total thermal energy.
There are three main temperature scales used today – Celsius, Fahrenheit, and Kelvin. The Celsius scale sets the freezing point of water at 0°C and the boiling point at 100°C. The Fahrenheit scale defines the freezing point of water as 32°F and the boiling point as 212°F. The Kelvin scale is an absolute temperature scale that uses the units of kelvins. It sets absolute zero, the theoretical lowest possible temperature, at 0 K. Water freezes at 273.15 K and boils at 373.15 K on this scale.
Relationship Between Heat and Temperature
Heat and temperature are related but distinct concepts. Heat refers to the total thermal energy in a system, while temperature indicates the average kinetic energy of molecules.
Adding heat to a system increases the temperature. For example, as you heat water on a stove, adding more heat increases the water’s temperature. The total heat energy rises as the water gets hotter.
Temperature correlates with the total heat energy. Higher temperatures mean more heat energy overall. This is because when molecules have more kinetic energy, the total thermal energy is greater.
While related, heat and temperature are not the same thing. Heat energy in a system can change independently of temperature in some cases. But in general, adding heat raises temperature, and higher temperatures indicate more heat energy.
Measuring Temperature
Temperature can be precisely measured using various types of thermometers that have been calibrated to different temperature scales. The most common scales used today are Celsius, Fahrenheit, and Kelvin.
Some of the most common thermometers include:
- Mercury thermometers – Based on the expansion of mercury with heat. Mercury is contained in a glass tube with degree markings.
- Alcohol thermometers – Similar to mercury but uses alcohol, which is less toxic. Mainly used for household applications.
- Infrared thermometers – Measure infrared energy emitted from an object. Allow non-contact temperature measurement.
- Thermocouples – Comprised of two dissimilar conductors that produce a voltage related to temperature differences.
- Resistance temperature detectors (RTDs) – Make use of the change in electrical resistance of some materials with changing temperature.
- Thermistors – Semiconductor-based sensors that exhibit a large change in resistance with temperature. Used in digital thermometers.
Thermometers must be properly calibrated to ensure accuracy. Traceability to national standards and periodic recalibration help thermometers maintain high precision temperature measurements over time.
Heat Transfer
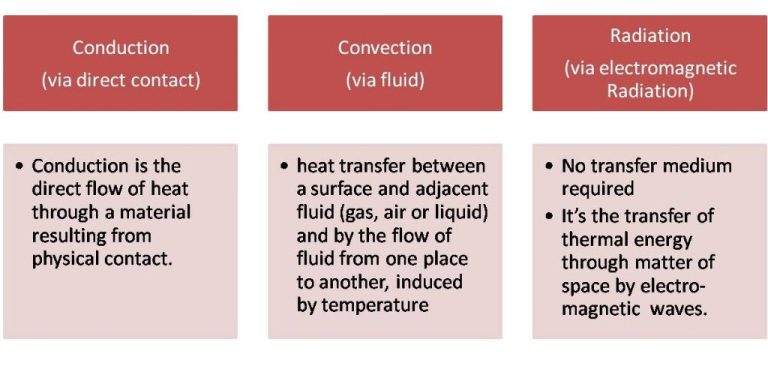
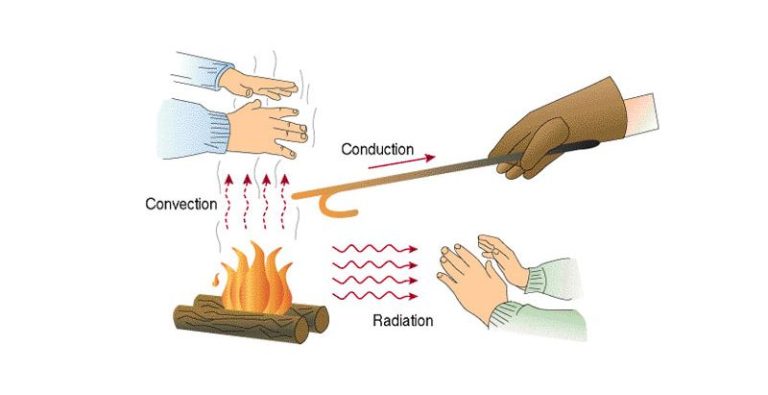
Heat energy can be transferred from one object to another in three main ways: conduction, convection, and radiation.
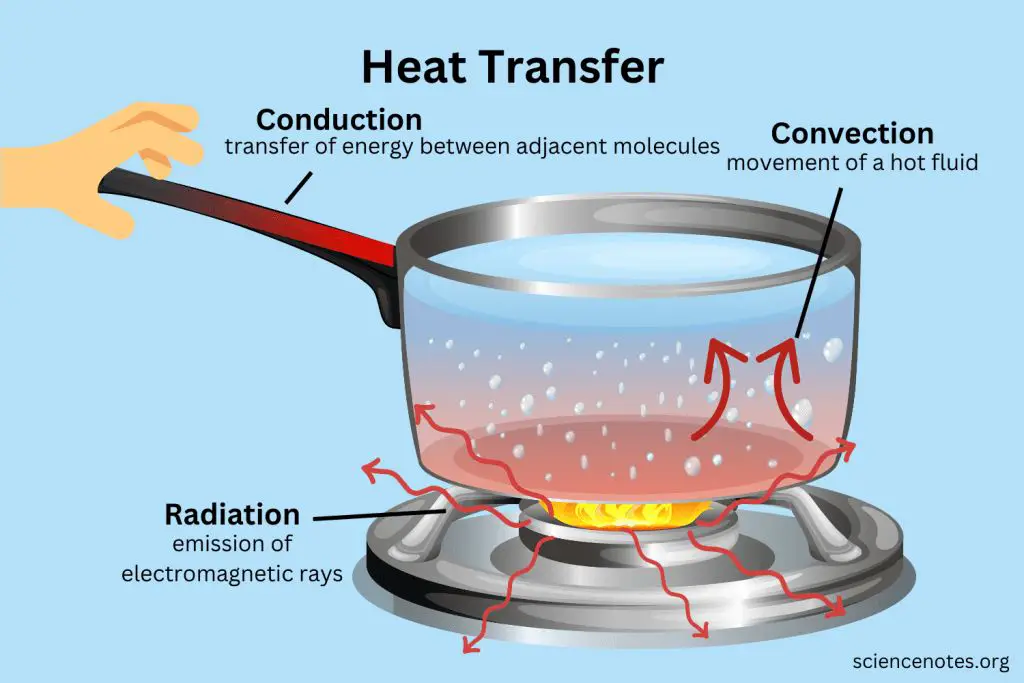
Conduction is the transfer of heat between objects that are in direct contact with each other. The better the conductor, the more rapidly heat will be transferred. Metals are good conductors. An example of conduction is a spoon getting hot after being left in a pot of boiling water.
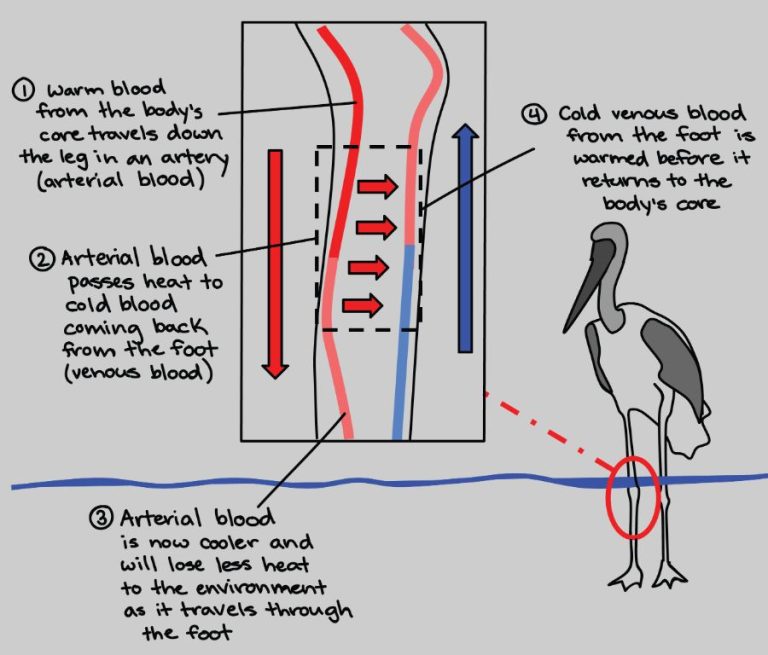
Convection is the transfer of heat by the movement of fluids. As the fluid is heated, it expands, becomes less dense, and rises. Cooler, denser fluid then moves to replace it, setting up a circulation pattern. This allows heat to be transferred away from the source. Convection currents are seen in pot of boiling water or the way heat circulates in a room.
Radiation is the transfer of heat via electromagnetic waves directly from the hot surface to anything in a line of sight. No direct contact is needed. The sun warming the Earth is an example of radiant heat transfer.
Effects of Heat
Heat energy has several important effects that shape both the natural world and human technologies. Some key effects of heat include:
Thermal Expansion
When materials are heated, the kinetic energy of their molecules increases. This causes the vibrations and distances between molecules to expand. As a result, almost all materials increase in volume as their temperature rises. This phenomenon is called thermal expansion and underlies everything from hot air balloons to bridges buckling on hot days.
Change of State
Heat energy can induce phase changes between solid, liquid, and gaseous states of matter. For example, applying heat to melt ice into water or boil water into steam. These changes of state require an input or output of energy called latent heat. Understanding phase changes and latent heat helps explain weather patterns and is harnessed in numerous engineering applications.
Chemical Reactions
Most chemical reactions can only occur at sufficient temperatures as the reactants need adequate energy to break bonds and reform into products. Heat controls reaction rates and yields. Many industrial processes rely on high-temperature chemical reactions, including smelting metals, generating electricity in thermal power plants, and manufacturing cement and glass.
Applications of Heat
Heat is useful for numerous applications in everyday life. Some of the most important applications involve using heat for cooking, powering engines and generating electricity, and facilitating industrial processes.
Cooking is one of the most common uses of heat. Applying heat to food through methods like baking, frying, grilling, etc allows food to be made safe and tasty for consumption. Modern society relies heavily on cooking with heat to prepare meals.
Heat is also harnessed as a power source for engines. Internal combustion engines in cars, planes, etc use the heat from burning fuel to create motion. Heat causes the fuel to expand rapidly and push pistons, which makes the engine run. The greater the heat, the more power can be produced.
Heat is used to produce electricity as well. Many power plants generate electricity by heating water to spin turbines connected to generators. Nuclear, coal, gas and even solar power plants rely on heat to ultimately create electrical current.
Finally, manufacturing industries use heat for tasks like melting, drying, and chemical reactions. Heat assists processes like metal production, chemical synthesis, food processing, and more. Modern civilization depends on harnessing heat energy for countless industrial purposes.
Heat and Life
Heat plays a vital role in supporting life on Earth. The human body must maintain a core temperature around 98.6°F (37°C) to function properly. We regulate our temperature through behaviors like sweating, shivering, and finding shade or warmth. If our core body temperature drops too low (hypothermia) or rises too high (hyperthermia), it can become life-threatening.
Heat energy flows through ecosystems and helps drive weather patterns, nutrient cycles, and other processes that allow life to thrive. Photosynthesis, for example, relies on the sun’s warmth to convert light into chemical energy. Heat also impacts habitats – many species can only survive within certain temperature ranges and will relocate or perish if their environment grows too hot or cold. Overall, heat provides the necessary energy for organisms to grow, reproduce and carry out the functions of life.
Fun Facts About Heat
Here are some fascinating fun facts about heat:
Hottest/Coldest Places
The hottest place ever recorded on Earth was Furnace Creek in Death Valley, California, where temperatures reached 134°F (56.7°C) in 1913. The coldest temperature on record is -128.6°F (-89.2°C), which occurred at Vostok Station in Antarctica in 1983.
Animals
The Sahara desert ant can tolerate temperatures up to 122°F (50°C) before suffering heat stroke. Emperor penguins can withstand temperatures as low as -76°F (-60°C) by huddling together.
Plants
The heat-loving cactus, native to hot deserts, can survive internal temperatures up to 149°F (65°C). The trembling aspen tree can withstand extreme cold down to -76°F (-60°C).
Records
The highest natural ground surface temperature ever recorded was 201°F (93.9°C) at Furnace Creek Ranch in Death Valley National Park in 1913. The lowest natural temperature directly recorded was -128.6°F (-89.2°C) at Vostok Station, Antarctica in 1983.
Importance of Heat Energy
Heat energy is essential for life on Earth. The sun’s radiation provides the heat that drives our climate and weather patterns. Without this incoming solar heat, the Earth’s average temperature would be about -18°C, far too cold to support life as we know it.
Heat energy plays a vital role in many natural cycles on Earth. It drives wind and ocean currents through convection, evaporated water to form clouds and rain, and plant transpiration and growth. Life as we know it depends on these processes.
Beyond sustaining life, heat is important for technology, industry, and human activities. We rely on heat for transportation, electricity generation, manufacturing, and more. Many industrial processes and machines convert heat into mechanical or electrical power.
Controlling heat is also crucial. Insulation keeps buildings warm in winter and cool in summer. Refrigeration preserves food and medicines that would otherwise spoil. Air conditioning allows people to live comfortably in hot climates.
Without properly regulating Earth’s heat budget, average temperatures would rise or fall dramatically. The greenhouse effect helps maintain hospitable temperatures, but human-caused global warming from excess greenhouse gases threatens to disrupt this balance and cause irreversible changes.