How Does Thermal Energy Flow?
Thermal energy refers to the total internal kinetic energy of molecules within a substance. This kinetic energy produces the perceptible effects of heating and cooling. Thermal energy can be transferred between objects through processes like conduction, convection and radiation.
All matter consists of atoms and molecules that are constantly moving and colliding with each other. The total kinetic energy from this molecular motion is thermal energy. When two objects at different temperatures come in contact, thermal energy will spontaneously flow from the warmer object to the cooler one until they reach thermal equilibrium. The transfer and flow of thermal energy is responsible for many everyday phenomena.
Conduction
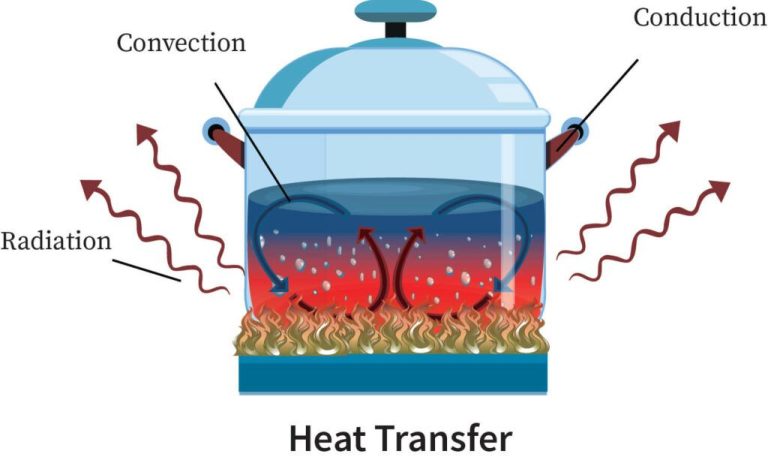
Conduction is the transfer of thermal energy between substances that are in direct contact with each other. It occurs when heat flows from a region of higher temperature to a region of lower temperature by direct contact of molecules.
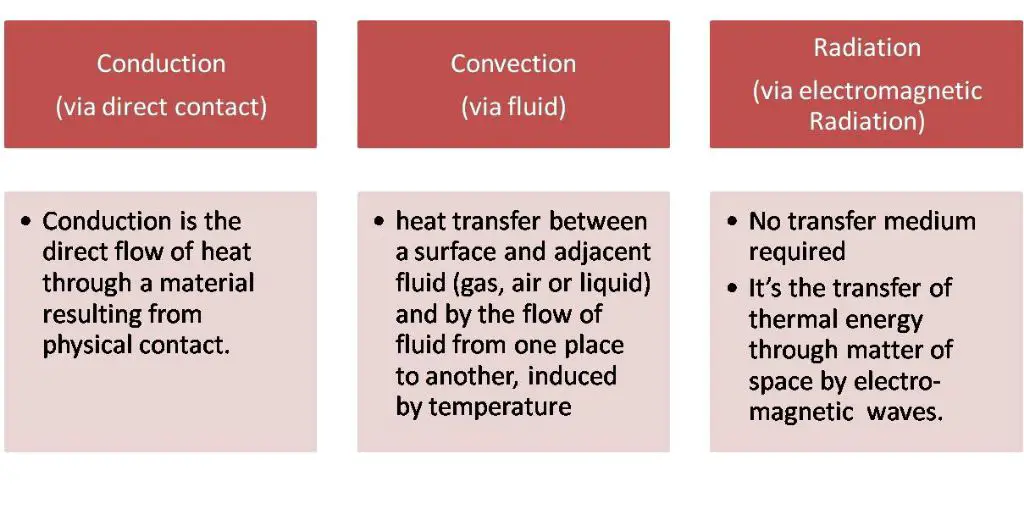
In conduction, thermal energy is transferred by the motion of molecules and electrons. As molecules and electrons interact, kinetic energy is transferred from the more energetic particles to the less energetic ones. Heat flows from warmer areas to cooler areas until thermal equilibrium is reached.
Materials like metals are good conductors of thermal energy. Their atomic structure allows heat to easily flow through them. Metals have free electrons that can carry thermal energy from one atom to another. Examples of good conductive materials include copper, aluminum, and steel.
Poor conductive materials like wood, plastic, and rubber have molecular structures that resist heat flow. Their electrons are not free to move easily, so heat transfer is slow. Poor conductors have low thermal conductivity and act as insulators.
Convection
Convection is the transfer of thermal energy by the movement of fluids or gases. When a fluid is heated, it expands and becomes less dense. The warm fluid then rises due to buoyancy forces, while the cooler, denser fluid sinks to take its place. This movement and circulation of fluid transfers heat energy from warm areas to cooler areas through the physical movement of the heated fluid.
A common example of convection is the circulation of air in a room being heated. The air nearest the heating source becomes warmed first. This less dense warm air rises, while cooler air moves in to take its place, creating circulation currents. The moving air transfers the thermal energy around the room. Convection currents are also responsible for the heating of air in the atmosphere and liquids like water when heated.
Convection can only occur in fluids, that is liquids and gases. It does not occur in solids, since the molecules in solids are not free to move and circulate.
Radiation
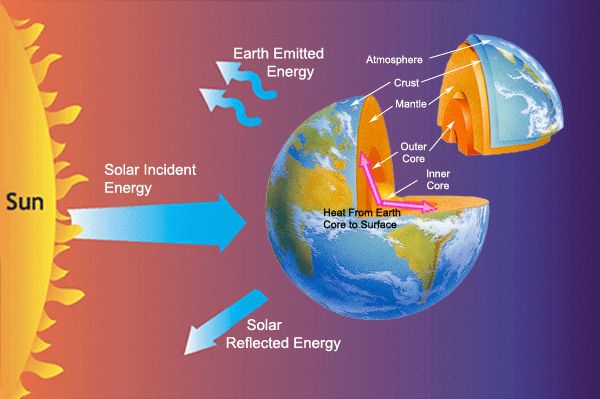
Radiation is the transfer of thermal energy by electromagnetic waves. All objects emit electromagnetic radiation related to their temperature. Thermal radiation does not require particles to transfer heat, unlike conduction and convection. Instead, thermal radiation transfers energy across space or a vacuum through electromagnetic waves.
The hotter an object is, the shorter the wavelength and higher the frequency of the electromagnetic waves it emits. For example, a red hot object radiates visible light while cooler objects emit infrared radiation. The sun produces a broad spectrum of electromagnetic radiation from radio waves to x-rays. The sun’s light and heat reach Earth’s atmosphere and surface as thermal radiation, warming our planet.
The rate of radiant heat transfer depends on the temperature of the emitting object. The Stefan-Boltzmann law describes the power emitted from a blackbody radiator per unit surface area. Real objects such as the sun do not radiate heat as perfectly as blackbodies, so their emissivity factor must also be considered.
Heat Transfer Rates
The rate of heat transfer refers to how quickly or slowly thermal energy is transferred between objects or systems. There are several factors that affect the rate of heat transfer:
Temperature Difference
The greater the temperature difference between two objects, the faster heat will be transferred between them. Heat flows from higher temperature to lower temperature. For example, an object at 100°C will transfer heat faster to an object at 20°C compared to an object at 90°C.
Surface Area
Objects with larger surface areas can transfer heat faster than objects with smaller surface areas. Doubling the surface area can double the heat transfer rate. This is because there are more sites for heat to be transferred across the boundary.
Material Properties
The thermal conductivity of materials affects how quickly they can conduct heat. Metals like copper and aluminum have high thermal conductivity and transfer heat rapidly. Insulators like wood and plastic have low conductivity and transfer heat slowly.
Distance
The distance heat must travel affects the transfer rate. Heat transfer decreases as distance increases because there are more opportunities for heat loss along the way. Bringing objects closer together allows for faster heat transfer.
In summary, larger temperature differences, more surface area contact, conductive materials, and shorter distances promote faster heat transfer rates between objects or systems.
Real-World Examples
Thermal energy transfers through conduction, convection, and radiation can be observed constantly in the world around us. Here are some everyday examples:
Conduction:
- Touching a hot stove burner results in thermal energy being conducted through the metal and into your skin.
- Wrapping your hands around a hot cup of coffee allows conduction to transfer thermal energy from the cup into your hands.
- Resting your bare feet on a cool tile floor conducts thermal energy from your feet into the floor.
Convection:
- Hot air rising from a radiator, causing convection currents that circulate warm air around the room.
- Opening an oven door resulting in a rush of hot air leaving the oven due to convection.
- The motion of boiling water as hotter, less dense regions rise through colder, denser regions.
Radiation:
- Feeling the warmth of the sun’s rays shining down, as heat radiates from the sun to the earth.
- Sitting in front of a fireplace allows the heat from the fire to radiate out and warm you up.
- Using a microwave oven that cooks food using radiant heat energy.
Applications
Thermal energy transfer has many practical applications in everyday life and technology. Here are some examples:
Insulation
Insulation materials like fiberglass and foam are specifically designed to slow down heat transfer through conduction. By trapping air pockets, insulation creates resistance to heat flow and helps keep buildings warmer in winter and cooler in summer.
Heating and cooling systems
HVAC (heating, ventilation and air conditioning) rely on principles of thermal energy transfer. Furnaces and air conditioners use convection to circulate hot and cold air. Radiators and baseboard heaters transfer warmth through radiation.
Cooking
The heating element of an electric stove transfers heat to a pot mainly through conduction. Inside the pot, convection currents distribute the warmth evenly. Ovens cook food using radiant heat from the baking coils.
Clothing and textiles
The materials we use for clothing and blankets leverage thermal properties. Down feathers trap air for insulation. Wool fibers allow slow conduction. Reflective space blankets minimize radiation loss.
Understanding thermal energy flows allows us to engineer solutions for managing temperature in many facets of everyday life.
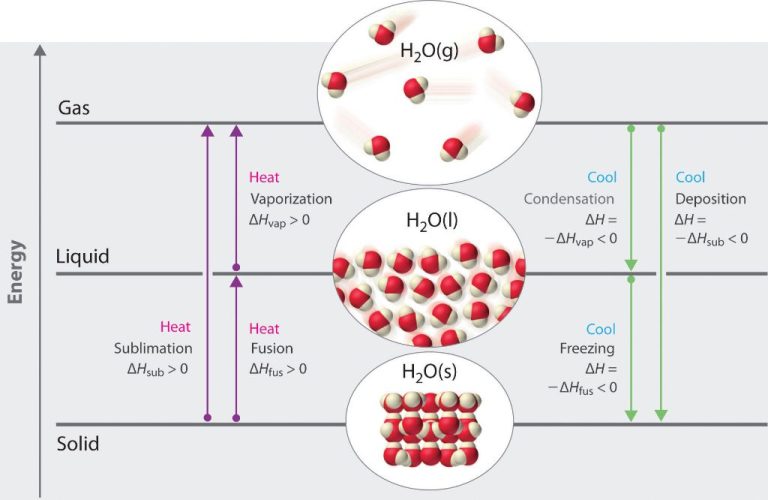
Effects on Matter
Thermal energy transfer can affect the state of matter of a substance. The states of matter are solid, liquid, gas and plasma. When thermal energy is added or removed from a substance, it can cause a phase change from one state of matter to another.
For example, when a solid substance like ice is heated by thermal energy transfer, it absorbs the thermal energy, causing the molecules to move faster and spread apart. As more thermal energy is added, the ice will eventually reach its melting point temperature and undergo a phase change to a liquid state (water).
Similarly, if a liquid is cooled by thermal energy transfer to the surroundings, it will lose thermal energy, causing the molecules to slow down and get closer together. At some point, the molecules become rigidly structured and the liquid undergoes a phase change to a solid state.
Thermal energy transfer can also cause phase changes between liquid and gas states through evaporation and condensation. When a liquid is heated, molecules can gain enough thermal energy to escape as a gas. And when a gas is cooled, it can condense back into a liquid state.
So in summary, thermal energy flow affects matter by causing phase changes between solid, liquid, gas and plasma states. The addition or removal of thermal energy alters the molecular structure and motion of a substance.
Related Concepts
Thermal energy is closely related to several other scientific concepts including thermodynamics, heat, and temperature:
Thermodynamics: Thermal energy flows from high temperature to low temperature objects according to the laws of thermodynamics. The movement of thermal energy is driven by differences in temperature between objects, and thermodynamics describes how this heat transfer occurs in nature and machines.
Heat: Heat refers specifically to the transfer of thermal energy between objects, while thermal energy is the total kinetic energy of molecules within an object. Thermal energy flows as heat from higher temperatures to lower temperatures.
Temperature: Temperature measures the average kinetic energy of molecules in an object. Objects with higher temperatures have faster molecular motion and can transfer more thermal energy to cooler objects. Temperature differences drive the flow of thermal energy as heat.
Understanding how thermal energy relates to these other concepts provides greater insight into how heat flows in the natural world and technical systems.
Conclusion
To summarize, thermal energy flows through conduction, convection, and radiation. Conduction is the transfer of heat between objects in direct contact, like a spoon heating up in a hot cup of soup. Convection is the movement of heat through fluids, such as hot air rising or water currents in the ocean. Radiation is the emission of electromagnetic waves from all objects, which allows the sun to warm the Earth. The rate of heat transfer depends on many factors like temperature difference, surface area and material.
Understanding how thermal energy flows is crucial for many scientific and engineering applications. It allows us to build efficient heating systems in homes, develop insulating materials, predict weather patterns, and much more. The universal nature of heat transfer impacts everything from cooking food to the climate of our entire planet. By studying conduction, convection and radiation, we gain a deeper understanding of the world around us.