What Form Of Energy Is Transferred From A Higher Temperature?
Heat is a form of energy that can be transferred from one object or system to another as a result of a temperature difference. Specifically, heat will flow spontaneously from a higher temperature object or system to a lower temperature one. This heat transfer changes the internal energy of both systems involved. Understanding the mechanisms of heat transfer and the direction of flow is key for many applications in thermodynamics and energy systems.
Definition of Heat
Heat refers to the transfer of thermal energy between systems or objects that are at different temperatures. Thermal energy is the kinetic energy associated with the random motion of atoms and molecules in matter. All matter contains thermal energy, and the amount is dependent on the temperature – higher temperatures mean greater kinetic energy at the atomic level.
When two objects at different temperatures come into contact, thermal energy will spontaneously transfer from the hotter object to the colder object. The hotter object has greater atomic/molecular motion, so its atoms/molecules collide with and transfer some of their energy to the atoms/molecules of the colder object. This process continues until the two objects reach thermal equilibrium and are at the same temperature.
So in summary, heat is the net transfer of thermal energy driven by a temperature difference between systems or objects. It flows from hot to cold until temperature equalization.
Temperature Difference Causes Heat Transfer
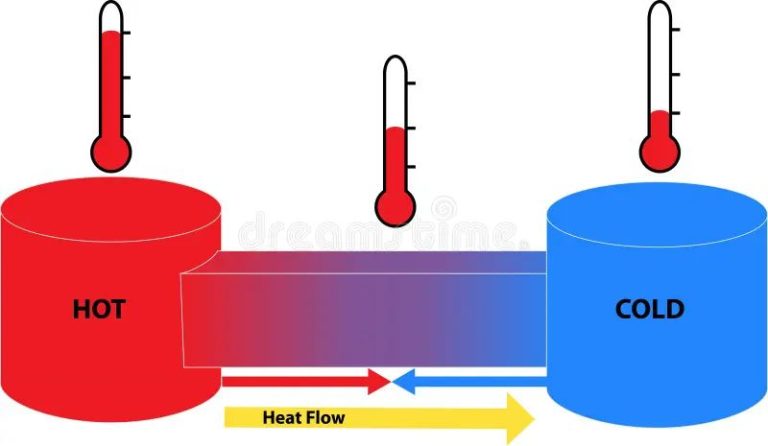
Heat transfer is driven by temperature differences. When two objects are in contact or proximity with each other, the object with the higher temperature will transfer heat to the object with the lower temperature until both objects reach thermal equilibrium and are at the same temperature.
This occurs because higher temperature corresponds to higher thermal energy, meaning faster molecular motion. When a higher temperature object comes in contact with a lower temperature object, the higher kinetic energy molecules collide with the lower kinetic energy molecules, transferring some of their energy during the collisions until equilibrium is reached.
The greater the temperature difference between the two objects, the faster the rate of heat transfer. Maximum heat transfer occurs when the temperature difference is greatest. As the temperatures equalize, the rate of heat transfer decreases.
This is why hot coffee cools down when left on the counter. The coffee starts at a high temperature but the room is at a lower temperature. Heat transfers from the hot coffee into the cooler room air until the coffee reaches room temperature.
Forms of Heat Transfer
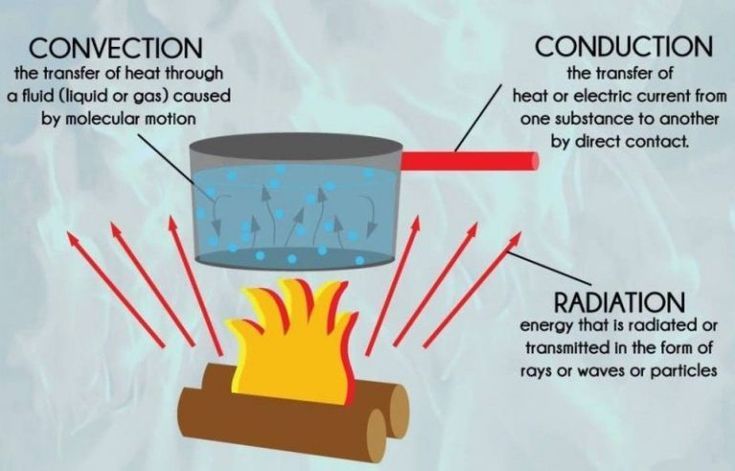
There are three main forms of heat transfer: conduction, convection, and radiation. Heat transfer occurs whenever there is a temperature difference between objects or systems. The warmer object will transfer thermal energy to the cooler object until they reach thermal equilibrium and are at the same temperature.
Conduction
Conduction is the transfer of heat between objects or systems that are in direct contact with each other. It occurs when atoms and molecules with higher kinetic energy collide with and transfer energy to adjacent atoms and molecules with lower energy. Metals are good conductors of heat because their free electrons can easily transport thermal energy.
Convection
Convection is the transfer of heat by the motion of fluids. It occurs between a solid surface and the adjacent liquid or gas in motion. The warmer fluid rises while the cooler fluid sinks, causing circulation. Convection can be natural, driven by temperature differences, or forced, via fans or pumps.
Radiation
Radiation is the transfer of heat via electromagnetic waves or photons. It can travel through empty space and does not rely on particle collisions. All objects emit thermal radiation related to their temperature. Radiation is the only form of heat transfer that can occur in a vacuum without relying on matter to transfer the heat.
Conduction
Conduction is the transfer of heat between substances that are in direct contact with each other. It occurs when faster moving atoms and molecules interact with slower moving atoms and molecules, transferring energy between them. Metals are good conductors because their atoms readily share vibrational energy.
Some examples of conduction include:
- Touching a hot stovetop pan – the heat transfers from the pan to your hand.
- Wrapping your hands around a warm cup of coffee – the ceramic mug conducts heat from the hot liquid to your hands.
- Putting on a sweater – the wool fibers conduct your body heat and insulate you.
Conduction works across solid materials, or between solids and fluids. It’s an efficient form of heat transfer that’s dependent on the temperature difference between the substances in contact.
Convection
Convection is a form of heat transfer that occurs between a surface and a moving fluid like a liquid or gas. As the fluid moves over the surface, it carries away heat energy from hotter areas and redistributes it to cooler areas. Hot air rising is a common example of convection. The hot air becomes less dense and rises while the cooler air sinks to take its place, creating a continuous circulation of air and heat transfer.
For example, the heating and cooling system of a house relies on convection. Hot air from a furnace rises through air vents and ducts, heating different rooms in the process as it circulates. Meanwhile, cool air gets drawn into the furnace, where it is reheated before being circulated again.
Convection occurs in cooking as well. As heat is applied to the bottom of a pot, the liquid at the bottom gets hotter than the liquid at the surface. This makes the hot liquid less dense so that it rises to the top while the cooler liquid sinks to the bottom to be reheated. The continuous circulation distributes the heat throughout the liquid.
In the atmosphere, convection creates wind as hot air rises and cooler air rushes to replace it. The rising hot air cools and sinks again, creating convection cells that transfer heat around the planet.
Radiation
Radiation is the transfer of heat energy through electromagnetic waves or photons. Unlike conduction and convection, radiation does not require direct contact between the heat source and the receiving object in order to transfer heat. Instead, the emitting object radiates thermal energy in the form of electromagnetic waves, which travel through space until they are absorbed by another object. All objects with a temperature above absolute zero emit thermal radiation.
Radiation can transfer heat energy over long distances as the waves can travel through empty space. The intensity of radiation emitted depends on the temperature of the object – the hotter an object, the more thermal radiation it emits. Examples of radiative heat transfer include:
- The warmth felt from a fireplace across the room
- The sun heating up the Earth across millions of miles of empty space
- An incandescent light bulb heating up its surroundings
- Microwave and infrared heating in ovens
- The Earth radiating heat energy into space to cool itself
Radiative heat transfer does not need a medium to travel through. It also occurs in a vacuum and can transfer heat over very long distances.
Heat Transfer Direction
Heat naturally flows from hotter objects to cooler objects. When two objects are at different temperatures, heat will transfer from the object at the higher temperature to the object at the lower temperature until they reach thermal equilibrium (both objects reach the same temperature). This occurs because higher temperature corresponds to higher thermal energy, so heat flows “downhill” from high to low thermal energy.
The direction of heat transfer is always from a higher temperature to a lower temperature. Hot cups of coffee cool down because heat transfers from the hot coffee to the cooler surrounding air. Ice cubes melt because heat transfers from the warmer room temperature air to the colder ice. An oven heats up food because heat energy transfers from the hotter oven air to the cooler food. This spontaneous and irreversible flow of heat is driven by the second law of thermodynamics, which states that the entropy of isolated systems always increases over time.
In summary, heat naturally transfers from higher temperature objects to lower temperature objects until thermal equilibrium is reached. This is a fundamental principle of thermodynamics that governs all heat transfer processes.
Applications
Heat transfer has many practical applications in the real world. Here are a few examples:
Cooking – Heat is transferred from the stove or oven to the food. Conduction occurs where the pan touches the stove, convection circulates hot air in the oven, and radiation transfers heat from the heat source to the food.
Heating and cooling systems – HVAC systems use conduction in the form of heat exchangers, convection through blowing air, and radiation through infrared heaters to transfer heat in buildings.
Internal combustion engines – Engines rely on heat transfer to convert thermal energy into mechanical work. Heat moves from burning fuel into the metal engine parts through conduction and then onto the working fluid (air or water) through convection.
Solar water heaters – Radiation from the sun hits a solar collector, heating up a fluid inside that then transfers heat through convection and conduction into the water supply.
Heat sinks – Computer processors and power electronics use heat sinks to transfer heat away through conduction. Fins provide additional surface area for convection and radiation.
Refrigeration – Heat pumps and refrigerators move heat from a colder reservoir into a hotter one, transferring heat against the natural direction through input of external work.
Conclusion
In conclusion, heat is the transfer of thermal energy from a higher temperature object or system to a lower temperature one. Heat naturally flows from hot to cold through conduction, convection, and radiation. Conduction transfers heat through direct molecular contact, convection relies on the circulation of fluids, and radiation works through electromagnetic waves. No matter the transfer method, heat will always spontaneously move from higher to lower temperatures until equilibrium is reached. This fundamental principle of thermodynamics drives everything from small scale combustion engines to the Earth’s global climate patterns. Understanding how and why heat flows down temperature gradients provides great insight into many natural and engineered processes.