What Does Adding Heat Mean?
Heat and temperature are related but distinct concepts. Heat refers to the total kinetic energy of molecules while temperature measures the average kinetic energy of molecules.
Adding heat to a substance increases the total kinetic energy of its molecules. This added energy can produce several effects:
- Increased molecular motion and vibrations
- Thermal expansion as particles take up more space
- Phase changes from solid to liquid to gas as particles break free of one another
- Increased rates of chemical reactions as reactants collide more frequently
- Rise in temperature as the average kinetic energy per molecule increases
In summary, adding heat increases the energy of a substance on a molecular level, leading to various macroscopic changes.
Heat Transfer
Heat transfer refers to the transfer of thermal energy between substances due to a temperature difference. There are three main mechanisms of heat transfer:
Conduction
Conduction is the transfer of heat between substances that are in direct contact with each other. It occurs when atoms and molecules with higher kinetic energy collide with and transfer energy to neighboring atoms and molecules with lower energy. Metals are good conductors of heat.
Convection
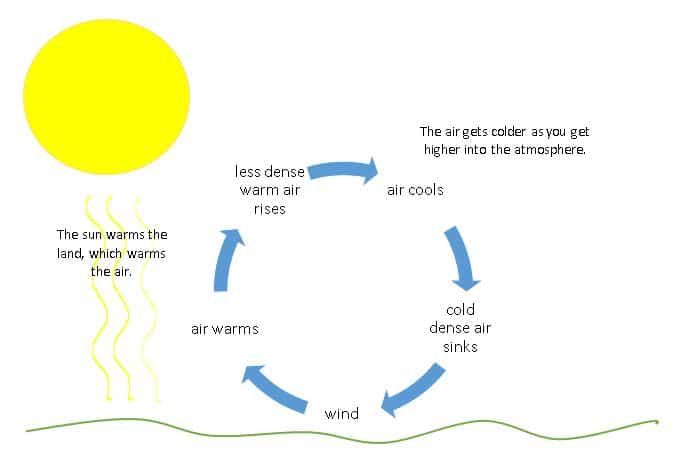
Convection is the transfer of heat by the movement of fluids. It occurs when heated particles with high energy in a liquid or gas move and take the place of cooler particles with lower energy. This causes circulation of heat energy. Convection currents play an important role in weather and ocean patterns.
Radiation
Radiation is the transfer of heat via electromagnetic waves. All objects emit infrared radiation depending on their temperature. Hotter objects emit more radiation than cooler objects. Radiation does not require particles to be in contact – it can travel through vacuums. The sun’s energy reaches Earth through radiation.
Increasing Molecular Motion
When heat is added to a substance, it causes the molecules of that substance to move faster. This is because heat is a form of energy, and when energy is transferred to the molecules, it gives them more kinetic energy to move around.
This increase in molecular motion happens in solids, liquids and gases. In solids, the molecules vibrate more rapidly in place as thermal energy is added. In liquids, the molecules can move around within the liquid more freely. And in gases, the molecules speed up and move much faster as heat increases their kinetic energy.
So in summary, no matter what state of matter a substance is in, adding heat will increase the molecular motion and kinetic energy of its molecules. This is because heat provides energy that enables the molecules to vibrate, rotate and move more vigorously.
Thermal Expansion
When heat is added to matter, the molecules gain kinetic energy and start moving faster and farther apart. This causes an increase in volume, which is known as thermal expansion. Thermal expansion occurs in solids, liquids and gases, but to varying extents.
In solids, the molecules are closely packed in an orderly structure. As heat is added, they vibrate more intensely against the intermolecular forces binding them together. This causes the solid to expand in size. For example, railroad tracks experience expansion during hot summer days and must have gaps to accommodate this expansion.
In liquids, molecules have greater freedom of motion and are farther apart than in solids. Heating a liquid increases the molecular motion, pushing the molecules slightly farther apart and expanding the volume. An example is the rising level of liquid in a thermometer as the temperature increases.
Gases consist of molecules with large spaces between them. Adding heat boosts their kinetic energy and causes them to spread out much more than solids or liquids, resulting in significant expansion. Hot air rises because it is less dense than surrounding cooler air. This effect is harnessed in hot air balloons to provide lift.
Thermal expansion is an important consideration in fields like construction, engineering, and manufacturing. Properly accounting for expansion and contraction from temperature changes prevents damage and malfunctions in materials and devices.
Change of State
One of the most noticeable effects of adding heat is the ability to change the phase or physical state of a substance. With sufficient heat energy, a solid can be transformed into a liquid, and a liquid into a gas. This is because heat provides the kinetic energy required to break the intermolecular bonds holding the substance’s molecules together in a rigid formation.
For example, by adding heat, solid ice can be melted into liquid water. If enough heat is continually added to the water, eventually the water molecules will gain enough energy to transition into a gaseous state (water vapor). The reverse also holds true – removing heat can cause gases to condense into liquids, and liquids to freeze into solids.
Each substance has defined temperatures at which these phase changes between solid, liquid, and gas will occur. The amount of heat required depends on the strength of intermolecular forces. Stronger molecular bonds in a substance will require more heat energy to overcome.
Understanding phase changes due to adding or removing heat is crucial for many chemical and industrial processes. It also explains phenomenons we observe in nature, such as water condensing from vapor to liquid water when cooled, or liquid water freezing into ice.
Increased Chemical Reactions
One of the most significant effects of adding heat to a substance is that it speeds up the rate of chemical reactions. Chemical reactions involve the breaking and formation of molecular bonds, and these processes require energy to take place. By adding heat, more energy gets transferred into the molecules participating in the reaction. This additional energy causes the molecules to move and vibrate faster, collide more frequently, and react more readily. It’s like giving the molecules a boost to overcome the energy barrier for the reaction.
A simple example is cooking an egg. The proteins in the egg undergo denaturation and coagulation reactions when heated, causing the egg to become solid. These reactions occur much faster at higher temperatures. Food chemistry abounds with many examples of heat accelerating chemical changes like the Maillard reaction, caramelization, and starch gelatinization. Even body temperature helps drive the thousands of essential biochemical reactions that occur within our cells.
Outside of food and biological contexts, heat also speeds up many important industrial chemical processes from cracking petroleum in oil refineries to synthesizing ammonia for fertilizers. Controlling temperature is crucial in chemistry, because every 10°C increase in temperature typically doubles the rate of reaction. The effect of heat on reaction kinetics is governed by mathematical laws like the Arrhenius equation. Overall, adding heat significantly increases the rate of chemical reactions, which is a fundamental mechanism by which energy gets transferred at the molecular level.
Temperature Changes
When heat is added to a substance, the temperature of that substance increases. This is because temperature is a measure of the average kinetic energy of the molecules in a substance. Adding heat adds energy to the molecules, causing them to move faster and bump into each other more. This increased molecular motion corresponds to an increase in temperature.
For example, heating water on a stove steadily increases its temperature until it reaches boiling point. The added heat from the stove provides energy to the water molecules, increasing their vibrations and movements. This in turn raises the average kinetic energy of the molecules, which is observed as a rise in temperature on a thermometer.
The relationship between heat and temperature is consistent across all matter. Whether a solid, liquid or gas, adding heat will lead to an increase in molecular motion and therefore a rise in temperature. This principle underlies everything from cooking food to power generation in thermal power plants.
Energy Transfer
Heat is a form of energy transfer between objects, not a substance itself. When we say an object gains or loses heat, what we really mean is that thermal energy is being transferred to or from that object. This occurs due to interactions at the molecular level.
Heat flows spontaneously from higher temperature objects to lower temperature objects. The higher kinetic energy of molecules in the hotter object is transferred to the cooler object when the objects come in contact, increasing the temperature of the cooler object. This flow of thermal energy continues until both objects reach the same temperature.
For example, when a pot of water is heated on the stove, the burner provides thermal energy that is absorbed by the water, increasing the kinetic energy of the water molecules. The water continues absorbing this heat energy until it reaches the same temperature as the heating element. The direction of heat flow is always from hot to cold.
Everyday Examples
We can see the effects of adding heat play out around us every day. Here are some common examples:
Cooking
Cooking relies on adding heat to food. As heat is added, the molecules in the food gain energy and start moving faster. This allows chemical changes and reactions to occur, changing the taste, texture, and appearance of food. For example, heat causes raw egg proteins to denature and coagulate into solid cooked egg.
Engines
Most engines like car engines rely on heat to function. Fuel is burned inside the engine, releasing a large amount of heat energy. This heat causes the molecules in the fuel and air to expand rapidly, creating pressure that pushes the pistons and enables the engine to do mechanical work.
Weather Patterns
Heating from the sun is the driving force behind many weather patterns. As the sun heats up air molecules at the equator, the warm air rises and flows to cooler areas, creating wind currents. The sun also evaporates water, powering the water cycle through rain and snow.
Conclusion
Adding heat ultimately means transferring energy into a system or substance, leading to an increase in molecular motion. The increased vibration, rotation and collisions of molecules manifests in key effects like thermal expansion, changes of state, faster chemical reactions, and rising temperatures. The physics of heat transfer underlies many everyday phenomena, from cooking food to powering engines. In summary, adding heat energizes molecules and atoms, driving physical and chemical changes that enable processes essential to life, technology, and nature.