What Does Heat Energy Flow From To?
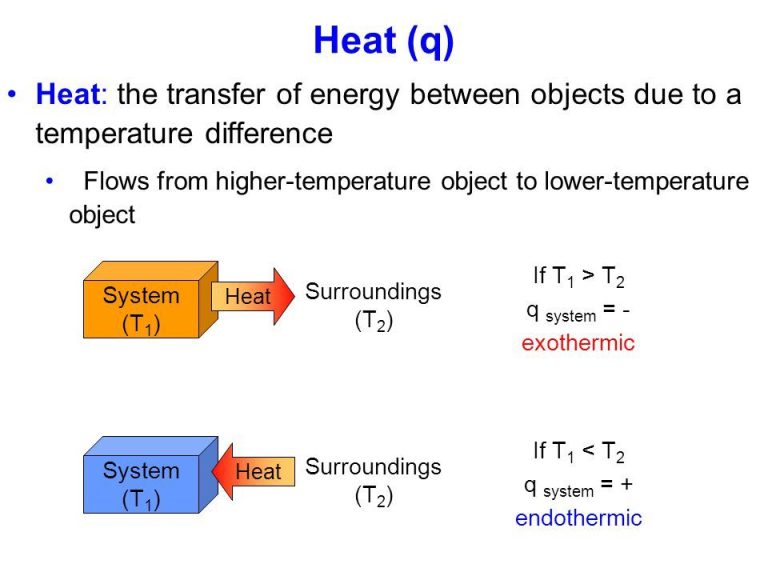
Heat energy transfer, also known as heat flow or heat transfer, refers to the transfer of thermal energy between objects or systems that are at different temperatures. This process tends to move heat from a hotter object to a colder one, until they reach the same temperature.
There are three main mechanisms by which heat energy can be transferred between objects:
- Conduction: The direct transfer of thermal energy between objects in physical contact without any flow of matter.
- Convection: Heat transfer through the motion of fluids (gases or liquids).
- Radiation: Heat transfer via electromagnetic waves directly between objects, without requiring physical contact.
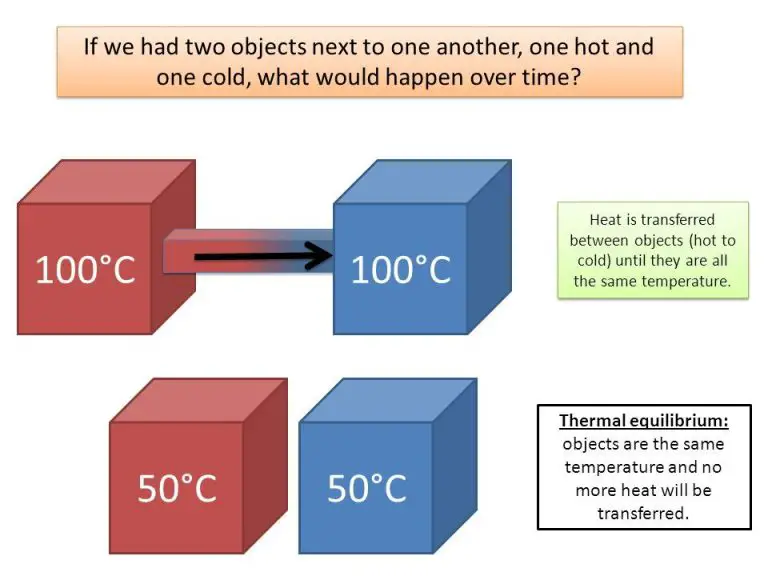
In general, heat will spontaneously flow from objects at a higher temperature to objects at a lower temperature. This process continues until both objects reach the same temperature, known as thermal equilibrium.
Conduction
Conduction is the transfer of heat energy between objects that are in direct physical contact. It occurs when faster moving molecules collide with slower moving molecules, transferring kinetic energy between them. Metals are good thermal conductors because their molecules can easily transfer kinetic energy.
Examples of conduction in everyday life include:
- Touching a hot stove causes your skin to get hot quickly as heat transfers through direct contact.
- Wrapping your hands around a hot mug warms your hands up.
- Putting a spoon in a hot cup of soup warms up the metal spoon.
Factors that affect the rate of conductive heat transfer include:
- Temperature difference – Larger temperature differences cause faster heat transfer.
- Thermal conductivity of materials – Metals conduct heat quicker than nonmetals.
- Thickness of materials – Thinner materials conduct heat faster.
- Surface area – More contact surface area allows faster conduction.
Convection
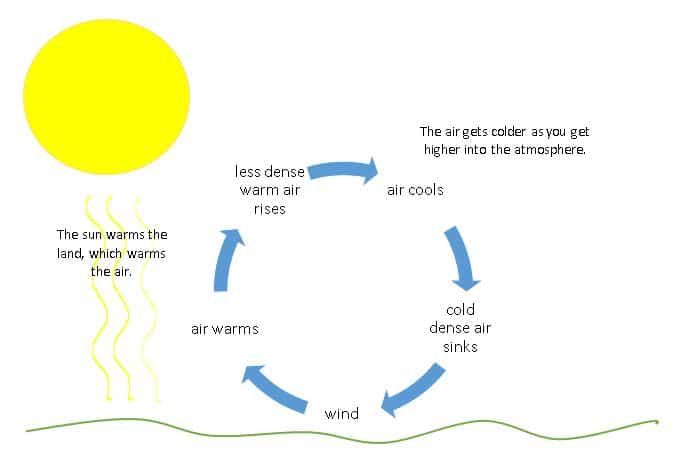
Convection is the transfer of heat by the movement of hot fluids. When a fluid is heated, it expands, becomes less dense, and rises while the cooler denser fluid sinks. This circulating motion allows heat to be transferred through liquids and gases. Convection currents are the flows that transfer heat using this mechanism.
Some examples of convection are:
- Hot air rising above a radiator and cooler air sinking.
- Lava lamps where the hot wax rises and falls as it cools.
- Boiling water where the hot water at the bottom rises while the cooler water sinks.
Convection plays a major role in weather patterns and ocean currents. The sun heats the Earth’s surface during the day causing warm air to rise and cool air to sink creating breezes and winds. At night, the Earth’s surface cools and the process reverses. In the ocean, cold dense water sinks at the poles and warmer water circulates globally forming major current patterns.
Radiation
Radiation is the transfer of heat energy by electromagnetic waves or photons. All objects emit some level of radiant energy, with the amount depending on their temperature. Hotter objects emit more radiation than cooler ones. Unlike conduction and convection, radiation does not require direct contact between objects or a medium to transfer heat. Instead, the energy is emitted in the form of photons or electromagnetic waves that can travel long distances through empty space before being absorbed. There are many examples of radiative heat transfer in everyday life:
- The warmth of the sun’s rays on your skin is caused by radiant energy emitted by the sun and absorbed by your body.
- Heat radiating from a fireplace that you can feel across the room.
- Heat inside an oven that cooks food without direct contact.
The rate of radiative heat transfer depends on the color and texture of surfaces. Dark, rough surfaces tend to absorb more radiant energy and light, smooth surfaces reflect more. For example, asphalt on roads absorbs and radiates more heat energy from the sun than concrete sidewalks, causing it to reach higher temperatures. Similarly, a black car left in the sun gets hotter than a white one. Understanding and controlling radiative heat transfer through surface colors and textures has many applications in science, engineering and everyday life.
Heat Flows from Warmer to Cooler Objects
The second law of thermodynamics states that heat energy will always spontaneously flow from a hotter object to a cooler object. This law underlies the fundamental behavior of heat transfer.
For example, when you place an ice cube into a hot cup of coffee, the heat from the hot coffee will flow into the colder ice cube, causing the ice to melt. The coffee does not spontaneously get colder while the ice cube gets hotter. Rather, the heat flows from hot to cold until both objects reach the same temperature, a state known as thermal equilibrium.
This principle applies to all types of heat transfer including conduction, convection, and radiation. For conduction, heat will flow through a material from the hotter end to the colder end. Convection relies on warmer fluids rising and cooler fluids sinking, enabling heat to be transferred away from hot surfaces. Thermal radiation works as infrared rays travel from hotter objects outward toward cooler surfaces.
In all cases, heat moves in one direction only – from warmer objects to cooler objects. This fundamental behavior allows us to transfer heat in useful ways, such as heating a house or cooling a processor chip. But it also places limits on what heat transfer can achieve, since heat will not spontaneously flow from cold to hot.
Measuring and Quantifying Heat Flow
Heat energy is measured in a variety of units depending on the system. The main units used are:
- Calories (cal) – This is the amount of heat energy needed to raise 1 gram of water 1 degree Celsius.
- Joules (J) – The joule is the SI unit for energy. 1 calorie is equal to 4.184 joules.
- British Thermal Units (BTU) – 1 BTU is the amount of heat needed to raise 1 pound of water 1 degree Fahrenheit. 1 BTU is equal to 1055 joules.
Instruments used to measure heat flow include:
- Thermocouples – Used to measure temperature differences which can quantify heat flow rates.
- Calorimeters – Measure changes in temperature to calculate heat flow into or out of a system.
- Heat Flux Sensors – Quantify heat flow per unit area, useful for measuring heat transfer through materials.
Some examples of quantifying heat flow are:
- Measuring the heat transfer through an insulating material in BTUs/hr/sq.ft.
- Using a calorimeter to measure the energy in calories absorbed or released in a chemical reaction.
- Determining the rate of heat loss in watts from a heated metal plate to its surroundings.
Applications of Controlling Heat Flow
The ability to control heat flow is extremely important in many engineering systems and thermal management applications. By manipulating how heat transfers between objects, engineers can optimize efficiency, safety, and performance.
Some common methods of controlling heat flow include:
-
Heat Sinks – These are materials or devices that absorb and dissipate heat from another object by maximizing surface area to improve convection and radiation. Heat sinks are commonly used to cool electronic components and systems.
-
Insulation – Insulating materials slow down heat conduction through substances. Insulation is crucial for maintaining desired temperatures, conserving energy, and protecting objects from excessive heat transfer.
-
Heat Pipes – Heat pipes are sealed tubes containing liquids that can transfer heat rapidly along the pipe between hot and cold regions. The liquid vaporizes at the hot end, carries heat, and then condenses at the cooler end.
-
Reflective Surfaces – Using polished or mirror-like surfaces reduces heat absorption from radiation sources. This is why space suits, satellites, and spacecraft have shiny exterior coatings.
Each of these technologies relies on an understanding of conduction, convection, and radiation to control the flow of heat energy. Insulation slows conductive heat transfer, heat sinks optimize convective and radiative transfer, and heat pipes leverage phase changes to move heat efficiently. The physics of heat transfer allow engineers to strategically speed up, slow down, or redirect heating and cooling within a system.
Thermal Equilibrium
Thermal equilibrium refers to the state where two objects that are in contact with each other no longer exchange heat energy. When two objects at different temperatures are placed together, heat flows from the warmer object to the cooler one until they reach the same temperature. At this point, the objects are said to have reached thermal equilibrium because their temperatures have equalized and no more net heat transfer occurs between them.
For example, if you pour hot water into a cold glass, the water will warm the glass and the glass will cool the water until they both stabilize at the same intermediate temperature. They have reached thermal equilibrium. Similarly, your body reaches thermal equilibrium with the air temperature of a room after being inside for a while.
Several factors affect how quickly thermal equilibrium is reached between objects:
- Temperature difference – A larger temperature difference results in faster heat transfer until equilibrium.
- Materials and specific heat capacity – Materials like metals transfer heat faster than insulators like wood or plastic.
- Surface area – More contact surface area allows faster heat transfer.
- Convection – Moving fluids transfer heat faster.
- Conduction – Better thermal conductivity allows faster heat transfer through a material.
Understanding thermal equilibrium allows us to engineer systems by choosing materials and designs that either speed up or slow down the rate of heat transfer. This principle is applied in many technologies from construction materials to heat sinks for electronics.
Impacts of Heat Transfer
Heat transfer can have significant impacts on the environment, weather, climate, and the human body. One major environmental concern is heat pollution. The release of excess heat into the air, water, and soil from industrial processes, vehicles, and appliances can disrupt ecosystems. Heat pollution warms bodies of water, increasing algal blooms and decreasing oxygen levels which harms aquatic life. It also warms the air, disrupting weather patterns and exacerbating climate change.
Heat transfer affects weather and climate on a global scale. The greenhouse effect, for example, is driven by heat energy from the sun being trapped in the atmosphere by greenhouse gases. This extra absorbed heat alters air and ocean currents, influencing weather events like storms and precipitation patterns. Climate change also shifts temperature ranges out of equilibrium as more heat is retained on Earth.
Within the human body, heat transfer regulates our core body temperature. When we exercise, metabolic processes generate excess heat that must be dissipated through conduction, convection, and radiation to prevent overheating. Sweating, for instance, uses evaporative cooling to shed heat. Shivering generates heat through muscle contractions when we are cold. These examples demonstrate how heat flow is critical for maintaining homeostasis.
Conclusion
In summary, heat is a form of energy that flows from warmer objects to cooler objects until thermal equilibrium is reached. Objects will transfer heat energy through conduction, convection or radiation. Heat flows from higher temperature to lower temperature objects, and will continue transferring until both objects reach the same temperature.
Understanding how heat transfers between objects allows us to control and utilize it for many applications. We can insulate objects to slow heat transfer, or promote heat flow for heating and cooling systems. Controlling heat transfer has many impacts on fields like engineering, architecture, chemistry and physics.
In conclusion, the core concept to remember is that heat energy spontaneously flows from warmer objects to cooler objects. Mastering heat transfer principles allows us to predictably control this process and apply it in many beneficial ways across science and engineering.