Why Is Thermal Energy Unique?
Thermal energy, often referred to as heat energy, is a form of energy related to the kinetic energy of atoms and molecules. It is a fundamental concept in thermodynamics and enables the transfer of energy between substances based on temperature differences. In this article, we will briefly introduce thermal energy and outline the key points regarding its kinetic nature, transfer methods, effects on matter, real-world applications, comparisons to other energy types, and unique properties that set it apart.
What is Thermal Energy?
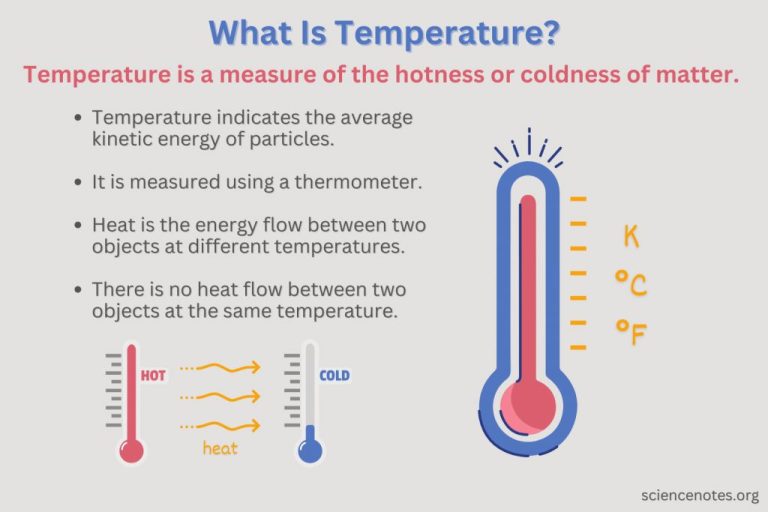
Thermal energy refers to the total kinetic energy of all the molecules within an object. It is often described as the internal energy that an object contains due to the motion and vibrations of its molecules and atoms. Thermal energy is directly related to the temperature of an object, which is a measure of the average kinetic energy of the molecules. The higher the temperature, the greater the molecular motion and thus the higher the thermal energy.
While related, thermal energy differs from heat. Heat is the transfer of thermal energy between objects due to a temperature difference. It flows from a body at higher temperature to a body at lower temperature. Thermal energy, on the other hand, exists within an object due to molecular motion regardless of its transfer. An object may gain or lose thermal energy during heat transfer, but the thermal energy itself remains distinct from the heat flow between objects.
In summary, thermal energy arises from molecular motion, temperature measures the average kinetic energy of molecules, and heat describes thermal energy transfer between objects due to temperature difference. Thermal energy is an important property of matter that manifests itself in heat transfer and effects on materials.
Kinetic Nature
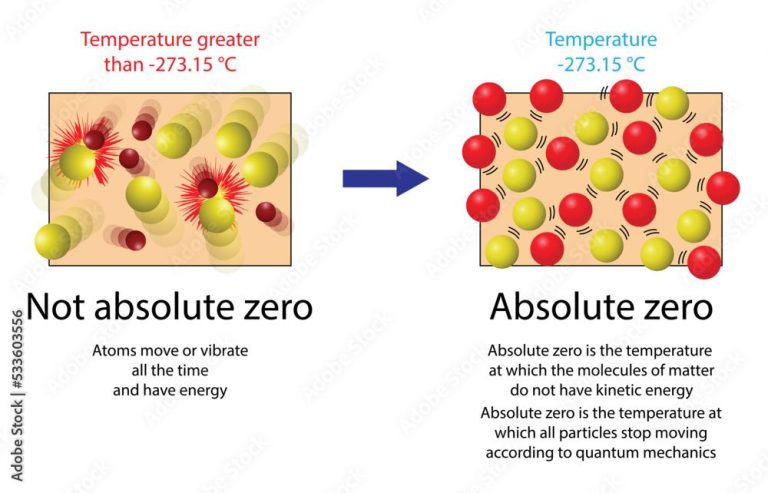
Thermal energy stems directly from the kinetic energy of atoms and molecules. Kinetic energy is the energy of motion – the movement and vibrations of atoms and molecules that make up matter.
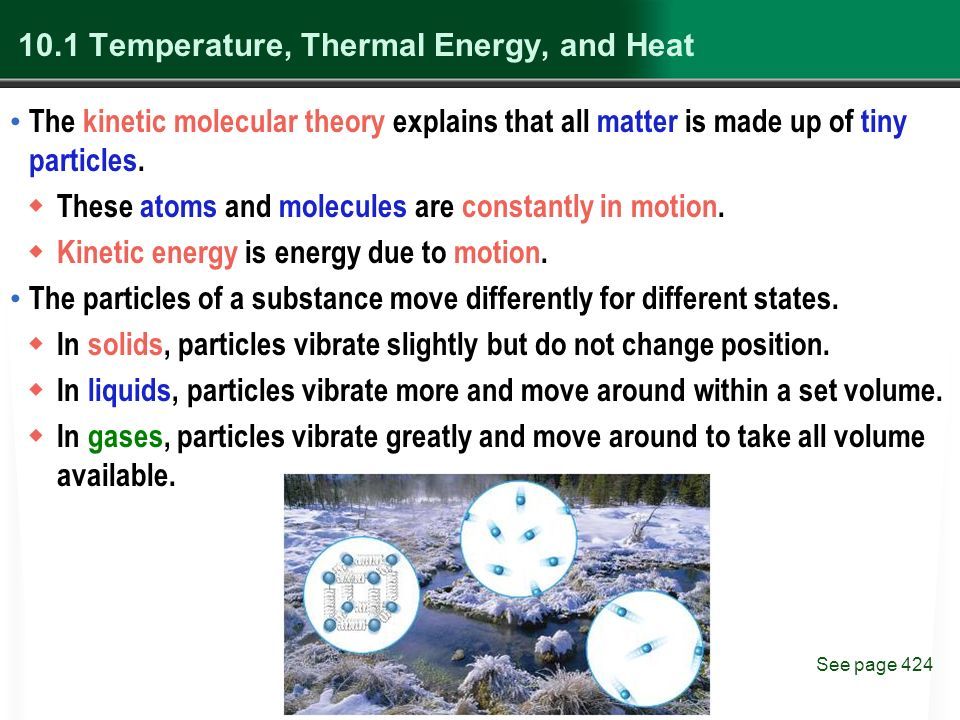
As the temperature of matter increases, the atoms and molecules move and vibrate faster as they gain kinetic energy. This increased motion and vibration of atoms and molecules is perceived as an increase in thermal energy. The thermal energy comes directly from the increased kinetic energy at the atomic level.
This kinetic nature is what makes thermal energy unique compared to other forms of energy. While other energy types such as electrical, chemical, nuclear, and electromagnetic relate to the structure, bonds, or radiation of matter, thermal energy arises specifically from the motion of atoms and molecules.
Understanding this kinetic origin of thermal energy also explains key concepts like heat transfer and thermodynamics. Heat flows from hotter to colder objects because of differences in kinetic energy at the atomic level. The laws of thermodynamics describe how this kinetic energy at the atomic scale affects large-scale properties and processes.
Transfer Methods
Thermal energy transfers between objects or regions that are at different temperatures. There are three main mechanisms of thermal energy transfer:
Conduction
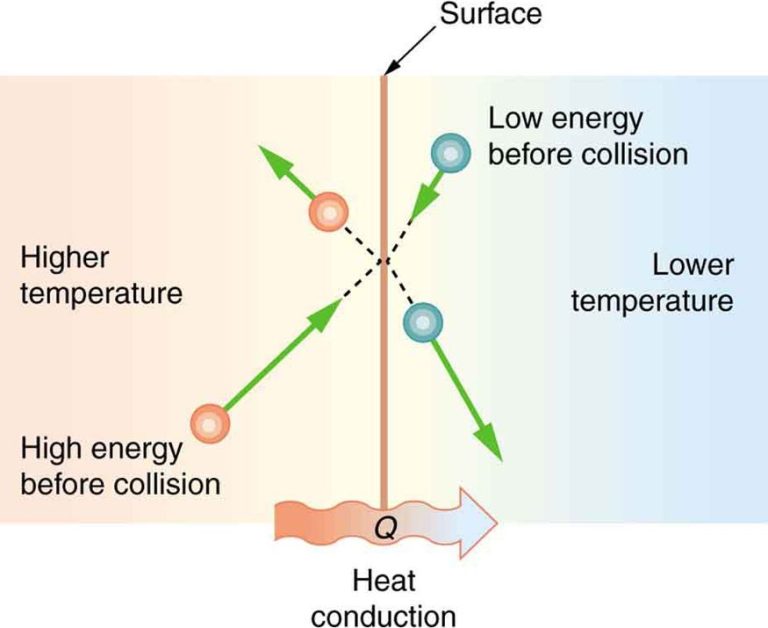
Conduction is the transfer of thermal energy between objects or regions that are in direct physical contact with each other. It occurs when atoms and molecules with higher kinetic energy collide with and transfer some of their energy to adjacent slower-moving atoms and molecules. Metals tend to be good thermal conductors.
Convection
Convection is the transfer of thermal energy by the movement of heated fluid. As the fluid (typically a liquid or gas) is heated, it expands, becomes less dense, and rises while cooler denser fluid sinks to take its place. This movement distributes thermal energy through the whole fluid. Convection occurs in the Earth’s mantle, the atmosphere, and hot liquids.
Radiation
Radiation is the transfer of thermal energy by electromagnetic waves. All objects emit electromagnetic waves due to their thermal energy. The higher the temperature, the shorter the wavelength and the higher the frequency of radiation emitted. Radiant energy can travel through empty space and pass through some materials. Our skin absorbs radiant energy from the sun and radiates it back into the environment.
Conservation of Energy
Thermal energy follows the fundamental law of conservation of energy. This means that when thermal energy is transferred from one system to another, the total amount of energy in the universe remains constant. While energy can change forms, it cannot be created or destroyed. For example, when you boil a pot of water on the stove, the thermal energy from the burning gas is transferred to the water, increasing its internal energy and raising its temperature. The total thermal energy before and after this transfer remains the same, even though the energy has shifted from the stove to the water.
The concept of conservation of energy is extremely important when analyzing thermal energy transfers and transformations. Whether looking at heating and cooling systems, chemical reactions, or biological processes, the energy input will equal the energy output when properly accounted for. There may be some energy lost to the surroundings, but the net change in the system will align with conservation of energy principles. This reliable, fundamental law allows scientists to quantitatively study thermal energy transfers with a high degree of certainty.
Effects on Matter
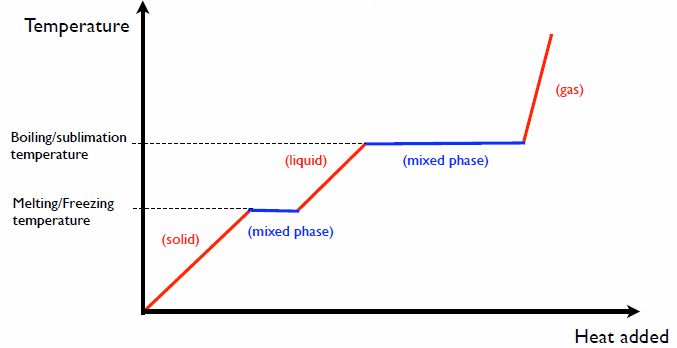
Thermal energy has a unique ability to alter the physical state of matter by adding or removing heat. For example, adding thermal energy can melt solids into liquids and vaporize liquids into gases. Removing thermal energy can cause gases to condense into liquids and liquids to freeze into solids.
This occurs because thermal energy affects the kinetic energy of molecules. Solids have lower molecular kinetic energy, keeping molecules bonded rigidly together. Adding thermal energy increases molecular motion, weakening bonds and allowing molecules to move more freely as a liquid or gas. Removing thermal energy slows molecular motion, allowing bonds to reform and molecules to arrange into orderly structures as solids.
The effects of thermal energy on matter also enable phase transitions – distinct physical changes between solid, liquid, and gas states. For example, freezing water into ice and boiling water into steam are both phase transitions driven by adding or removing thermal energy. No other form of energy can directly cause these dramatic alterations to molecular structure and mobility.
Thermal energy’s capacity to melt, evaporate, freeze, and condense matter makes it exceptionally useful for manipulating the physical properties of substances. Many industrial processes rely on the application or removal of heat to shape and treat materials. Thermal energy’s effects on matter also play essential roles in natural processes across scientific disciplines.
Real-World Applications
Thermal energy principles have many important real-world applications in fields like engineering, construction, and weather forecasting. Here are some examples of thermal energy concepts being applied:
- Buildings and insulation – Architects use principles of thermal conduction, convection, radiation and resistance to design effective insulation and HVAC systems that heat or cool buildings efficiently.
- Cooking – Cooking uses conduction on the stove and convection in ovens to transfer heat and transform ingredients.
- Internal combustion engines – Engines rely on thermal expansion of gases to generate power and motion.
- Solar power – Solar thermal systems use radiation from the sun to heat up water or other fluids that can generate electricity.
- Weather forecasting – Meteorologists study transfers of thermal energy in the atmosphere and oceans to model and predict weather patterns.
- Thermal imaging – Thermal cameras detect infrared radiation emitted by objects to create images based on heat signature and temperature.
As you can see, thermal physics drives many essential technologies and processes we rely on. Studying thermal energy gives insights into improving real-world systems.
Thermal Energy vs Other Energy Types
Thermal energy stands apart from other common forms of energy like chemical energy, electrical energy, nuclear energy, and more. Here’s a look at how it contrasts with these other types:
Chemical Energy: This is the energy stored in the bonds between atoms and molecules. Batteries and food are examples of stored chemical energy. While chemical reactions can release thermal energy, chemical energy itself is distinct in that it is stored in substances rather than directly relating to the kinetic energy of atoms/molecules.
Electrical Energy: Electricity involves the flow of electrons, which is different from the vibration of atoms that comprises thermal energy. While electrical current can generate heat, the energy itself stems from the movement of charge rather than kinetic molecular motion.
Nuclear Energy: Energy derived from nuclear fission or fusion involves changes within atomic nuclei. This is separate from the kinetic energy associated with thermal energy. However, fission and fusion processes do release significant amounts of heat.
Overall, thermal energy is unique in that it deals directly with the kinetic molecular energy of matter, separately from energy stored in atomic bonds, flowing charges, or nuclear binding forces. This distinctive characteristic leads to the specific methods of transfer and applications associated with thermal energy.
Unique Properties
Thermal energy has several unique properties that distinguish it from other forms of energy like electrical, chemical, nuclear, and gravitational potential energy.
First, thermal energy involves the kinetic energy of atoms and molecules. As temperature increases, the vibrational motion of particles becomes more intense. This atomic-scale kinetic nature makes thermal energy fundamentally different from potential energies which depend on position or chemical configuration.
Second, thermal energy can be transferred via conduction, convection, and radiation between objects and within materials. The ability for heat to be transmitted through solid, liquids, and gases allows thermal energy to spread in ways that other forms cannot.
Third, thermal energy is subject to thermodynamic laws like conservation of energy and entropy that dictate how it flows and transforms. These thermodynamic principles govern fundamental processes like heating, cooling, and phase changes in a unique manner.
Fourth, thermal energy affects matter in distinct ways, such as thermal expansion, changes in solubility, and shifts in reaction rates. The strong influence of heat on material properties sets thermal energy apart.
Fifth, thermal energy is universally experienced in daily life through heating/cooling systems, combustion engines, and weather. Practical applications relying on heat are unique and ubiquitous compared to uses of other energy forms.
In summary, properties like kinetic nature, transfer methods, thermodynamic laws, effects on matter, and real-world applications make thermal energy fundamentally unique compared to other forms of energy.
Conclusion
Thermal energy stands apart from other types of energy in several key ways. As we’ve covered, thermal energy is the kinetic energy associated with atomic and molecular motion. It is transferred via conduction, convection, and radiation between substances, converting into other forms in the process. While the total energy is always conserved, thermal energy affects states of matter uniquely. Substances expand when heated and contract when cooled. This allows for many practical applications, from sterilization to power generation. Thermal energy is in a constant state of flux, flowing from hotter to colder objects. This universal tendency towards equilibrium makes thermal energy vital for everything from weather patterns to cooking food. In summary, thermal energy is a fundamental form of kinetic energy that powers change and drives diversity in our universe. Its unique properties make it indispensable.