Why Can’T You Destroy Energy?
The Law of Conservation of Energy
The law of conservation of energy states that the total amount of energy in an isolated system remains constant. Energy cannot be created or destroyed, only transformed from one form to another (https://energyeducation.ca/encyclopedia/Law_of_conservation_of_energy). For example, when a light bulb is turned on, electrical energy is transformed into light energy and heat energy. The energy powering the light bulb is not destroyed, but rather changed into other forms of energy.
According to the law of conservation of energy, the total energy of an isolated system is conserved over time (https://en.wikipedia.org/wiki/Conservation_of_energy). This means that energy can change forms within the system, but the total amount of energy remains the same. For instance, chemical energy in gasoline can be converted into kinetic energy to propel a car forward. But the total amount of energy within the car system remains constant, assuming no energy escapes or enters from outside the system.
Forms of Energy
There are many different forms of energy that exist in the universe. The main forms of energy include:
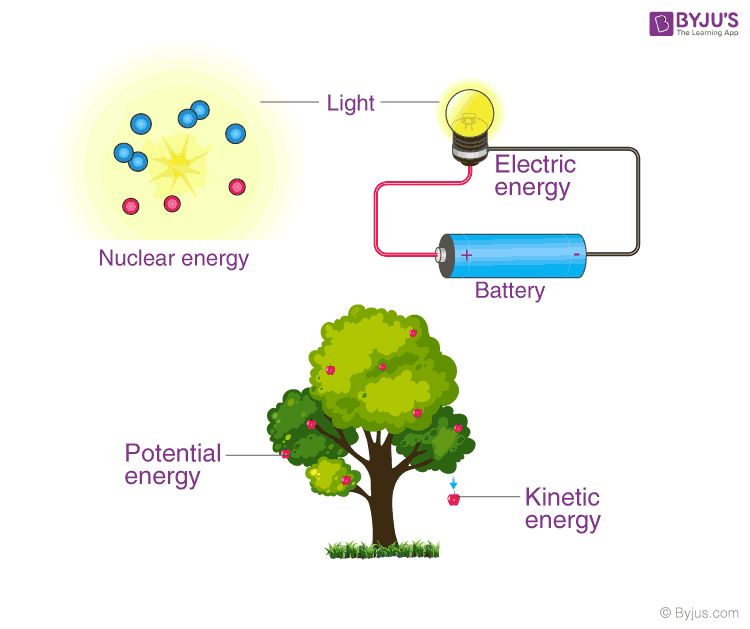
- Kinetic Energy – The energy possessed by an object in motion. Examples include the energy of a moving bullet, the motion of wind, or the vibration of atoms.
- Potential Energy – Stored energy possessed by an object due to its position or state. Examples include energy stored in a compressed spring, water held behind a dam, or the chemical bonds of molecules.
- Chemical Energy – Energy stored in the bonds between atoms that make up molecules. It is released or absorbed during a chemical reaction. Examples include energy stored in explosives, batteries, food, and fossil fuels.
- Thermal Energy – The internal energy of substances that arises from the random motion of particles that make up the substance. Heat is the transfer of thermal energy between substances.
- Nuclear Energy – Energy stored within the nucleus of an atom and released through nuclear reactions. Examples include nuclear power plants where fission reactions produce heat to generate electricity.
- Electrical Energy – Energy derived from electric charge or flow of electrons. Examples include energy in lightning bolts, batteries, and outlets.
- Radiant Energy – Energy transmitted by electromagnetic radiation such as light, radio waves, gamma rays, and X-rays.
These different forms of energy can be converted and transformed into other types. However, the total energy remains constant according to the law of conservation of energy.
Energy Transformations
Energy can change from one form to another through transformations. Some common examples of energy transformations include:
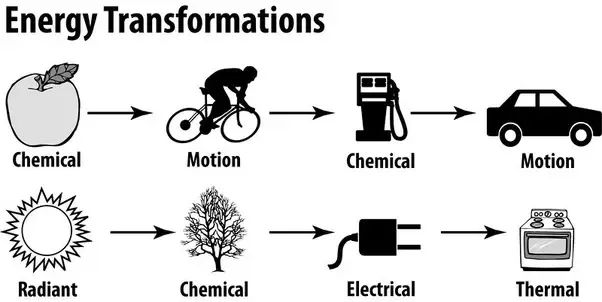
Chemical energy to kinetic energy – When gasoline combusts in a car engine, the chemical energy stored in the gasoline transforms into kinetic energy that propels the car forward. According to the Energy Conversion site by Solar Schools, chemical energy contained in food can also transform into kinetic energy in our bodies as we move.
Nuclear energy to thermal energy – Nuclear power plants rely on nuclear fission, where the nuclear energy stored in atoms transforms into thermal energy that can boil water to spin turbines for electricity generation.
Radiant energy to chemical energy – Plants rely on radiant light energy from the sun transforming into chemical energy during photosynthesis to grow and thrive.
These are just a few everyday examples of how energy transforms from one form to another while obeying the law of conservation of energy. The total energy before and after a transformation remains constant.
Energy Cannot Be Created
The law of conservation of energy states that energy cannot be created or destroyed – it can only be transformed from one form to another [1]. This fundamental law of physics is based on extensive scientific testing and thought experiments over centuries, establishing that energy must have originated somewhere – it cannot simply come into existence from nothing.
For example, imagine holding a ball at a height above the ground. When you drop the ball, gravitational potential energy is transformed into kinetic energy as the ball falls. The kinetic energy of motion is then converted into heat, sound and deformation energy when the ball hits the ground. But the total amount of energy remains constant – the energy wasn’t created from nothing when the ball dropped. It was converted from potential to kinetic.[2]
Many such real-world examples confirm that while energy can change forms, the total quantity of energy in a closed system always remains fixed. Energy must come from an existing source – it cannot simply be created from nothing. This scientific law underlies our modern understanding of physics and the nature of energy.
Energy Cannot Be Destroyed
According to the law of conservation of energy, energy can neither be created nor destroyed – only transformed from one form to another. This law, also known as the first law of thermodynamics, states that the total energy in an isolated system always remains constant.
For example, when a ball drops, its potential energy gets transformed into kinetic energy. The chemical energy stored in the bonds of gasoline gets transformed into mechanical energy and heat as the gasoline powers a car engine. In nuclear reactors, mass gets converted into huge amounts of thermal and electromagnetic energy. In all these examples, the total energy before and after the transformation remains the same.
The law of conservation of energy is considered to be one of the most fundamental laws in physics. It has been validated through repeated scientific experiments and observations across a wide range of scales and phenomena. While energy can change forms, the total quantity always remains fixed. This principle helps explain where energy came from in the early universe and provides a constant framework for studying energy dynamics in closed systems.
In summary, the first law of thermodynamics prohibits energy from being created or destroyed, only transformed between different forms. This conservation law underlies our quantitative understanding of energy and its transformations across the sciences.
(Source: https://www.scientificamerican.com/article/energy-can-neither-be-created-nor-destroyed/)
Attempts to Create Energy
Throughout history, there have been many attempts to create usable energy from nothing through the invention of perpetual motion machines. Perpetual motion machines are hypothetical devices that, once set in motion, would continue moving forever with no additional energy input 1. These attempts aim to produce endless motion, thereby creating energy in a continuous cycle. However, perpetual motion machines are impossible according to the law of conservation of energy.
The law of conservation of energy states that energy can neither be created nor destroyed, only transformed from one form to another 2. Perpetual motion machines would violate this fundamental law of physics by continually producing energy without any external source. The machines are designed to use the energy they generate to sustain their own motion, but this would require creating new energy, which is scientifically impossible.
Many ingenious designs for perpetual motion machines have been proposed and built, but all have failed to live up to their promise of limitless, self-sustaining energy generation. The impossibility of these machines stems from unavoidable energy losses due to friction, air resistance, and other inefficiencies inherent to physical systems. No real machine can avoid these losses, dooming all perpetual motion machines to eventual slowing and stopping.
Practical Applications
The law of conservation of energy has many important practical applications in physics, engineering, and other scientific fields. According to the law, the total amount of energy in a closed system remains constant over time. Energy can transform from one form to another, but it cannot be created or destroyed.
One example from engineering is the design of roller coasters. When a roller coaster car travels down a slope, it picks up speed due to the conversion of gravitational potential energy into kinetic energy. The kinetic energy is then partially converted into thermal energy due to friction in the wheels and air resistance. However, the total amount of initial gravitational potential energy is equal to the sum of the final kinetic, potential, and thermal energies. Engineers apply the law of conservation of energy to analyze and optimize the performance of roller coasters.
In chemistry, the law of conservation of energy is used to determine whether a chemical reaction can occur spontaneously. Reactions that release energy in the form of heat or light are spontaneous since the total energy of the products is lower than the reactants. On the other hand, reactions that absorb energy are non-spontaneous. The energy released or absorbed in a reaction can be calculated using the law of conservation of energy.
Physicists apply the law across all disciplines from thermodynamics to quantum mechanics. It is a fundamental principle that enables the calculation of the kinetic energy of particles in particle accelerators, the energy radiated by stars, and many other important physical phenomena. The law helps explain how energy flows and transforms within systems as diverse as living cells, electrical circuits, and galaxy clusters.
Overall, the law of conservation of energy is indispensable for understanding our universe on both large and small scales. It allows scientists and engineers to apply an energy accounting framework across many different situations.
Common Misconceptions
One of the most common misconceptions about the law of conservation of energy is that it means energy can be created from nothing. This is simply not true. The law states that energy cannot be created or destroyed – it can only be transformed from one form to another (Source).
For example, when gasoline is burned in a car engine, chemical energy in the gasoline is transformed into heat and kinetic energy that powers the car. The total amount of energy remains constant, even though the forms of energy change.
Another misconception is that consuming energy “destroys” or “uses up” energy. In reality, energy is conserved at all times. The energy we consume is simply converted to less useful forms like heat that dissipate into the environment. Energy is never destroyed, it just spreads out and becomes harder to use (Source).
The law of conservation of energy tells us it is impossible to destroy energy. Common misconceptions arise from a misunderstanding that energy can disappear or no longer exist. But the total amount of energy in a closed system always remains constant.
Philosophical Implications
The law of conservation of energy has profound philosophical implications. The idea that energy can never be created or destroyed connects to philosophical and spiritual perspectives about the nature of the universe.
Some philosophers have used the law of conservation of energy to argue for the existence of God. The cosmological argument, for example, states that the universe must have been created by an “unmoved mover” that exists outside of time and space. Since energy cannot be created, something eternal must have created the energy in the universe.
The law of conservation also supports the idea that energy is eternal and cyclical. Energy transforms between different states, but the total amount remains constant. This aligns with Eastern philosophies about the cyclical and interconnected nature of all things in the universe.
Ultimately, the indestructibility of energy points to deeper mysteries about the origins and workings of the cosmos. While physics can describe how energy behaves, the deeper question of why remains unanswered. The law of conservation provides hints to this philosophical puzzle but does not offer a complete solution.
Conclusion
In summary, the law of conservation of energy states that energy can never be created or destroyed, only transformed from one form to another. This universal law underpins all physical interactions and processes. Through examples and analysis, we’ve seen how energy dynamically changes forms – from potential to kinetic, chemical to electrical, etc. But the total quantity of energy remains constant.
The law of conservation of energy is one of the most fundamental laws of physics. It has been validated by countless experiments over centuries. There are no known exceptions to this law. While energy can change forms through various interactions, it cannot vanish or appear from nothing. The total energy of an isolated system always remains fixed.
This immutable fact has profound implications for our understanding of the universe. It means the total energy content of the universe is constant, despite all its dynamic transformations and exchanges. In essence, energy can only evolve into different manifestations – it cannot be created or destroyed. This conservation law will likely remain a cornerstone of physics for the foreseeable future.