What Is The Energy Transfer Of Energy?
Energy transfer refers to the movement of energy from one place or system to another. When energy is transferred, it changes from one form to another while the total amount of energy remains constant. This concept is described by the law of conservation of energy. Understanding energy transfer is fundamentally important in science and engineering because it allows us to trace where energy comes from, how it changes forms, and where it ultimately ends up. Knowing how energy moves and transforms helps us utilize it more efficiently in things like power generation, transportation, electronics, and more.
Forms of Energy Transfer
There are three main ways that energy can be transferred between objects or systems: heat transfer, work transfer, and radiation transfer.
Heat Transfer
Heat transfer occurs when two objects or systems at different temperatures come into contact, allowing energy to flow from the hotter to the colder body. This energy transfer continues until both objects reach the same temperature. Heat transfer can occur through conduction, convection or radiation. Conduction is the direct transfer of thermal energy between particles that are touching, such as a spoon heating up when placed in hot soup. Convection involves the movement of heated fluid particles transferring heat, like hot air rising or water currents warming up a pool. Radiative heat transfer involves electromagnetic waves, such as the warmth provided by sunlight.
Work Transfer
Work transfer involves energy being transferred when a force causes an object to move. For example, when you push a crate across the floor, your force does work to move the crate a certain distance. The amount of work equals the force multiplied by the distance. Work transfers occur in many mechanical and electrical systems.
Radiation Transfer
Radiation transfer is the emission and absorption of energy as electromagnetic waves or photons. This allows energy to be transferred over long distances without direct contact between source and receiver. For example, radiant energy from the sun can travel through the vacuum of space and be absorbed by objects like the Earth and plants. Other examples are X-rays, radio waves, and the heat of a campfire felt from a distance. No medium is required for radiation transfer to occur.
Heat Transfer
Heat transfer is the transfer of thermal energy from one object or system to another due to a temperature difference. The three main mechanisms of heat transfer are conduction, convection, and radiation.
Conduction is the transfer of heat between two solid objects in contact. It occurs when warmer molecules transfer kinetic energy to neighboring cooler molecules without any motion of the matter itself. Metals like copper and aluminum are good conductors of heat.
Convection is the transfer of heat by the movement of heated fluid. It occurs in liquids and gases when regions with higher temperature rise due to lower density, carrying heat with their motion. Natural convection occurs due to temperature differences while forced convection uses an external source like a fan or pump.
Radiation is the transfer of heat in the form of electromagnetic waves. All objects emit infrared radiation depending on their temperature. Heat is transferred when these waves strike another object. Radiation does not require a medium and can even occur in a vacuum.
Work Transfer
Work transfer occurs when energy is moved between objects through the application of force over a distance. There are two main types of work in the context of energy transfer:
Mechanical Work
Mechanical work happens when a force is applied to an object, causing it to move a certain distance. For example, when you push a box across the floor, you are doing mechanical work on the box. The amount of mechanical work done is calculated by multiplying the force applied by the distance moved. Mechanical work increases the kinetic energy of an object.
Electrical Work
Electrical work involves the movement of electric charges, such as electrons, from one point to another due to an electric field. For instance, when an electron moves through a wire in a circuit, electrical work is done. The amount of electrical work is calculated by multiplying the charge moved by the voltage. Electrical work can produce other forms of energy like light, heat, or motion.
Understanding work transfer is important for designing efficient systems and processes that convert energy from one form to another with minimal losses. Both mechanical and electrical work are central to many energy technologies in areas like transportation, power generation, and electronics.
Radiation Transfer
Radiation is the transfer of energy by electromagnetic waves. This can occur through light, radio waves, X-rays, and other types of radiation that are part of the electromagnetic spectrum. Radiation does not require a medium to travel through, and can even transfer heat through the vacuum of space.
One example of radiation transfer is the energy we receive from the sun. The sun produces vast amounts of electromagnetic radiation in the form of light and heat. This solar radiation travels through space until it reaches Earth’s atmosphere and surface. The radiation interacts with the molecules it encounters, transferring the sun’s energy for various uses like heating Earth’s surface and fueling photosynthesis in plants.
Other examples include radio waves for communication, microwaves for cooking food, and infrared radiation that allows night vision technologies to see in the dark. Different wavelengths of electromagnetic radiation have different properties and energy levels that determine how they can be utilized.
Overall, radiation transfer is a key process for distributing energy across vast distances through photons, waves and rays that can travel unimpeded through space or transparent media. This allows energy to be transferred in ways that do not rely on physical contact between matter.
Energy Conversion
The transfer of energy frequently involves the conversion of energy from one form to another. Some key forms of energy conversion include:
Chemical Energy
Chemical energy is stored in the bonds between atoms and molecules. This energy can be released in chemical reactions and converted into other forms such as heat, light, electricity or motion. Examples of chemical energy conversion include burning fuel in an engine or metabolizing food in cells.
Nuclear Energy
Nuclear energy is stored in the nucleus of atoms. It can be released in nuclear reactions and converted into huge amounts of heat or electricity. Nuclear power plants convert nuclear energy into electrical energy through fission reactions.
Electrical Energy
Electrical energy results from the flow of electrons. It is frequently converted into other types of energy such as light, heat or motion through electrical devices. Generators convert mechanical energy into electricity.
Thermal Energy
Thermal energy relates to the kinetic energy of atoms and molecules. It can be converted into mechanical energy to produce motion or electricity to power turbines and engines. The burning of fuels converts chemical energy into thermal energy.
Mechanical Energy
Mechanical energy is energy stored in objects by tension, compression or motion. It can be converted into electrical energy through generators or converted into thermal energy through friction.
Law of Conservation of Energy
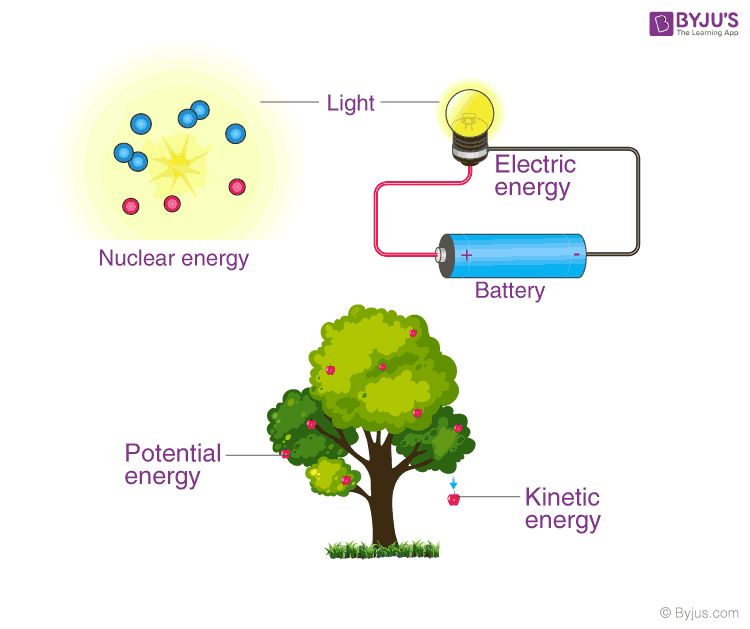
The law of conservation of energy is one of the most fundamental laws of physics. It states that energy can never be created or destroyed – it can only be transferred or transformed from one form to another. This means that the total amount of energy in an isolated system always remains constant.
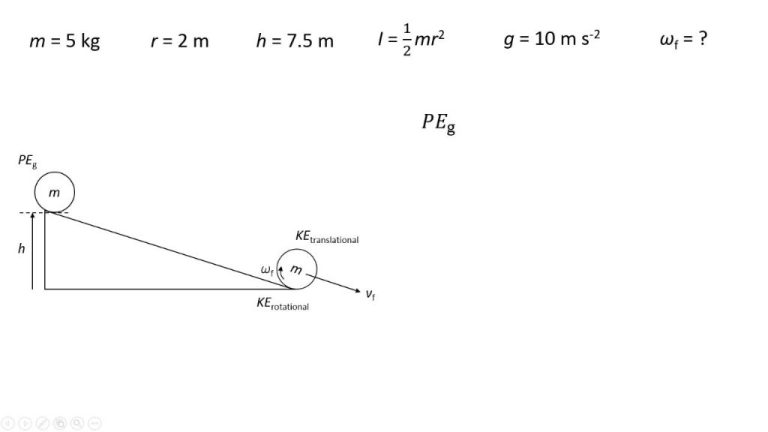
For example, when a pendulum swings, it transfers energy between kinetic energy and potential energy – but the total amount of energy within the pendulum system remains the same. When chemical energy in gasoline is converted into kinetic energy to move a car, no energy disappears. It just changes form. This law allows us to track where energy comes from and where it ends up.
The law of conservation of energy is crucial for understanding our universe on both large and small scales. It explains where the sun’s energy comes from and how energy flows through ecosystems. Engineers apply it to improve energy efficiency in vehicles, buildings and industrial processes. Overall, this fundamental law underlies all transfers and transformations of energy in our universe.
Real-World Examples
Energy transfers occur constantly in the natural world and in human technology. Here are some important real-world examples:
Photosynthesis
Photosynthesis in plants is an energy transfer process where light energy from the sun is converted into chemical energy stored in glucose molecules. This chemical energy is then transferred and used by the plant.
Digestion
In animals, the chemical energy stored in food is transferred and converted through digestion into a form that cells can use. Digestion breaks down large molecules into smaller ones that can be absorbed and metabolized.
Batteries
Batteries convert stored chemical energy into electrical energy through redox reactions. The electrical energy can then be used to power devices or converted into other forms of energy. Rechargeable batteries can reverse this process, taking in electrical energy and converting it back into chemical energy for storage.
Engines
Engines like those in cars and generators convert the chemical energy stored in fuel into mechanical energy or electrical energy through combustion. The energy transferred from the fuel allows the engine to perform work.
Improving Efficiency
There are several ways we can improve energy efficiency in our daily lives and reduce energy waste. Some examples include:
Insulation
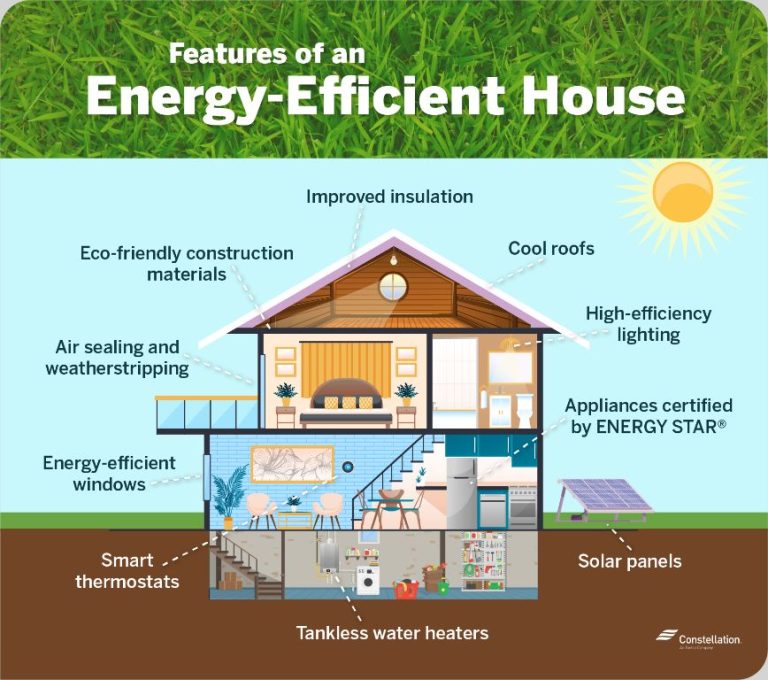
Adding insulation to walls, ceilings, attics, basements, and crawlspaces is one of the most effective ways to improve home energy efficiency. Insulation creates resistance to heat flow and reduces heat loss in winter and heat gain in summer. Good insulation also improves comfort and reduces noise.
LED Light Bulbs
Replacing incandescent and CFL light bulbs with LED bulbs can provide huge energy savings. LED bulbs use at least 75% less energy and last 25 times longer than incandescent bulbs. Switching entirely to LEDs in a home can reduce lighting energy use by 90%.
Hybrid and Electric Vehicles
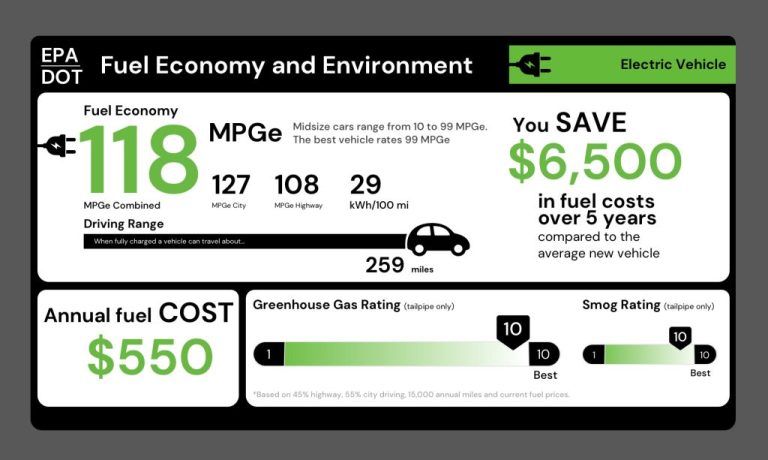
Driving a fuel efficient hybrid or fully electric vehicle reduces gasoline consumption and lowers emissions. Electric vehicles convert over 77% of electrical energy to power the wheels, whereas gas-powered cars only convert 12-30% of energy to power. Widespread adoption of electric vehicles could significantly reduce transportation energy usage.
With some smart energy efficiency improvements like added insulation, LED lighting, and electric vehicles, we can greatly reduce energy waste and consumption in our homes and daily transportation.
Conclusion
Energy transfer is an essential process that allows work to be done and life to be sustained. As we have seen, there are several forms of energy transfer including heat, work, and radiation. While energy can change forms, it is never created or destroyed according to the law of conservation of energy.
Understanding how energy transfers between objects and systems is crucial for improving efficiency. With growing energy demands worldwide, it is important that we find ways to transfer energy with minimal losses. This could involve enhancing technologies and processes to reduce waste heat and friction. Individuals can also contribute by being mindful of energy use in their daily lives.
Overall, the transfer of energy connects and sustains all systems on Earth and beyond. Though the core principles remain unchanged, there are always opportunities to fine-tune energy transfer processes for optimal efficiency.