What Processes Recycle Carbon?
Photosynthesis
Plants play a vital role in recycling carbon through the process of photosynthesis. During photosynthesis, plants absorb carbon dioxide (CO2) from the atmosphere through small pores called stomata on their leaves. The CO2 molecules combine with water from the soil and energy from sunlight to produce glucose sugar and release oxygen (O2) as a byproduct.
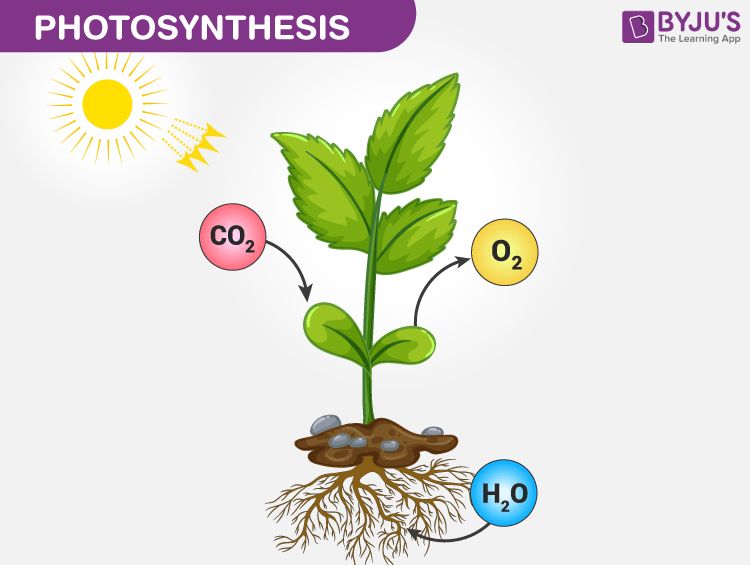
The chemical equation for photosynthesis is:
6CO2 + 6H2O + sunlight –> C6H12O6 + 6O2
This conversion of CO2 into plant matter is the first step in recycling carbon within the biosphere. Through photosynthesis alone, it’s estimated that plants absorb over 100 billion tons of CO2 per year globally, which helps offset some of the CO2 emissions from human activities. The glucose sugars produced during photosynthesis provide plants with the energy they need to grow. Some of this new plant matter may get consumed by animals or decompose, releasing CO2 back into the atmosphere. But a portion of plant biomass gets stored for longer periods as wood, roots, leaves, and soil organic matter. This temporary carbon storage makes the photosynthesis process an essential component of the global carbon cycle.
Respiration
Respiration is a process that recycles carbon dioxide in the environment. All animals, including humans, participate in respiration. The basic process involves breathing in oxygen and breathing out carbon dioxide.
During inhalation, oxygen enters the lungs and diffuses into the bloodstream. The oxygen is transported through the circulatory system to the body’s cells. Inside the cells, the oxygen is used along with glucose in a series of chemical reactions known as cellular respiration. This process converts the stored energy in glucose into a form that cells can use for growth, movement, and other functions.
A waste product of cellular respiration is carbon dioxide. This carbon dioxide diffuses into the blood and travels back to the lungs. When you exhale, the carbon dioxide leaves the body. The carbon atoms in the exhaled carbon dioxide originally came from organic molecules like glucose, which were produced by plants through photosynthesis. Therefore, respiration recycles the carbon atoms absorbed by plants back into the atmosphere as carbon dioxide, where they are available for reuse by plants.
The constant recycling of carbon between living organisms and the atmosphere by respiration and photosynthesis is called the carbon cycle. This crucial process allows carbon atoms to be reused indefinitely as they cycle through ecosystems on Earth.
Decomposition
Decomposition is a key process that recycles carbon back into the atmosphere and soil. When organisms such as animals, plants, fungi, and bacteria die, their organic matter is broken down through the process of decomposition. This happens in both terrestrial and aquatic ecosystems.
Decomposition is carried out by decomposer organisms such as bacteria, fungi, invertebrates like earthworms and insects, and scavengers. These organisms consume dead organic matter and break it down into simpler inorganic compounds such as carbon dioxide, water, and mineral salts.
As these decomposers break down complex organic molecules like cellulose and lignin found in dead plants, carbon dioxide is released as a byproduct. This CO2 is cycled back into the atmosphere and taken up by plants during photosynthesis. Decomposition therefore forms a critical linkage in the carbon cycle between life and death.
On land, decomposition happens on the surface, in the soil profile, and in aquatic systems like wetlands. The rate of decomposition is dependent on environmental factors like temperature, moisture and oxygen availability. In the ocean, decomposition happens more slowly due to colder temperatures and lack of oxygen at depth.
Human activities can impact decomposition rates. For example, deforestation and drainage of wetlands exposes more organic matter to decomposition. On the other hand, landfills slow down decomposition due to lack of oxygen. Understanding the role of decomposition in the carbon cycle can help manage carbon budgets and fluxes.
Ocean-Atmosphere Exchange
The ocean plays a crucial role in regulating the amount of carbon dioxide (CO2) in the atmosphere through the continuous exchange of CO2 at the ocean-atmosphere interface. Here’s how it works:
CO2 from the atmosphere dissolves into the surface layer of the ocean, a process known as air-sea gas exchange. The ocean acts as a massive carbon sink, absorbing and storing roughly 30% of annual global CO2 emissions.
Conversely, CO2 is also released back from the ocean to the atmosphere. As ocean waters circulate and mix, CO2-rich water from the interior ocean gets brought up to the surface and CO2 is transferred back into the air.
The direction and rate of CO2 exchange depends on the concentration gradient between the atmosphere and surface ocean. Higher atmospheric CO2 concentrations drive increased uptake by the ocean. Meanwhile, colder water temperatures allow for greater CO2 absorption.
Natural oscillations such as El Nino can temporarily reduce CO2 uptake by bringing deep, CO2-rich waters to the surface. Over the long-term, the ocean absorbs more CO2 than it releases, helping regulate Earth’s temperature and climate.
Chemical Weathering
Chemical weathering is a key process that recycles carbon dioxide from the atmosphere. It involves certain rocks and minerals chemically reacting with carbon dioxide to form new compounds like carbonates. This process starts when carbon dioxide from the air dissolves into rainwater, forming a weak carbonic acid. As this acidic water flows over rocks, it reacts with minerals like calcium silicates. The calcium silicates bind with carbon dioxide to form calcium carbonate minerals such as limestone. These carbonate minerals are stable and can lock away the carbon for thousands or millions of years. Over geologic timescales, weathering of rocks like basalts, granites and schists has removed massive amounts of carbon dioxide from the atmosphere through chemical weathering and carbonate formation. Currently, chemical weathering annually removes around 0.25 gigatonnes of carbon from the atmosphere. While this is small relative to human CO2 emissions, it illustrates the critical role of chemical weathering and carbonate formation as a key long-term carbon sink.
Carbon Capture & Storage
Carbon capture and storage (CCS) is a process that captures carbon dioxide emissions from sources like power plants and industrial facilities. The CO2 is then compressed and transported via pipeline to a storage site, where it is injected and stored deep underground.
There are several ways that CO2 can be captured from emission sources:
Post-combustion capture: This involves separating CO2 from the flue gases produced by combustion of fossil fuels or biomass after the fuel is burned. The CO2 is absorbed using chemical solvents like amines.
Pre-combustion capture: Here, the fossil fuel is processed in a gasifier to produce hydrogen and CO2. The two streams are separated, and the hydrogen is burned to generate power while the CO2 is captured.
Oxy-fuel combustion: This uses oxygen instead of air for combustion, producing a flue gas that is mainly CO2 and water vapor. The water vapor is condensed, leaving a highly concentrated CO2 stream that can be captured.
Once captured, the CO2 is compressed into a dense phase for easier transport. It is then transported via pipeline or ship to a suitable storage site.
Deep underground geological formations like depleted oil and gas reservoirs or deep saline aquifers are used for storing the injected CO2. Impermeable cap rock overhead traps the buoyant CO2 and prevents upward migration.
Biochar
Biochar is a process that converts plant matter such as tree branches, crop waste, and wood chips into charcoal, which can then be buried in soil. As the plant matter is heated in a low-oxygen environment, it transforms into charcoal, storing carbon that was previously in the atmosphere.
When biochar is mixed into soil, that carbon stays locked away instead of decomposing and releasing carbon dioxide back into the atmosphere. Biochar also enhances soil fertility by improving its ability to retain nutrients and water. The carbon in biochar can remain stable in soil for hundreds to thousands of years.
Creating and burying biochar is a carbon negative process – it actively removes carbon dioxide from the atmosphere. Widespread use of biochar could lead to net negative carbon dioxide emissions, counteracting some human emissions. The biochar can be produced through modern pyrolysis facilities or rudimentary stoves and kilns. Both small-scale subsistence farmers and large-scale commercial operations can utilize biochar to enhance soil while sequestering carbon.
Reforestation
Reforestation refers to planting trees in areas that were previously forested but cleared due to human activity such as logging or agriculture. Planting new trees helps pull carbon dioxide out of the atmosphere through photosynthesis and sequester the carbon in the trees and soil.
As trees grow, they absorb carbon dioxide from the air, using it to produce carbohydrates and build biomass through photosynthesis. About half of a tree’s biomass is carbon that gets stored in the trunk, branches, leaves and roots. This carbon gets locked up in the trees for the lifetime of the trees, which could be decades to centuries depending on the species.
In addition to the carbon stored in the above-ground tree biomass, reforestation also leads to increased carbon storage in the soil. Trees help build soil organic carbon by shedding leaves and fine roots that decompose and add organic matter to the soil. The soils of reforested areas can accumulate significant quantities of carbon over time.
Studies show that reforestation has excellent potential for absorbing and storing atmospheric carbon if done over large enough areas globally. One study estimated that reforestation has the potential to store over 200 gigatonnes of carbon by 2100 if global tree restoration goals are met. This would make reforestation one of the most effective carbon dioxide removal strategies.
Ocean Fertilization
Ocean fertilization involves adding nutrients like iron, nitrogen, or phosphorus to specific areas of the ocean in order to stimulate the growth of phytoplankton. Phytoplankton are microscopic algae that function as an integral part of the marine food web. They also play a key role in the ocean’s biological carbon pump, absorbing massive amounts of carbon dioxide through photosynthesis. When phytoplankton die, they sink to the ocean floor carrying that stored carbon with them. This effectively removes and “sequesters” carbon from the atmosphere.
By fertilizing the ocean, the goal is to boost phytoplankton blooms. More phytoplankton should lead to increased photosynthesis, and therefore a greater amount of carbon dioxide absorption. Researchers have estimated that large-scale and sustained ocean fertilization could potentially sequester billions of tons of carbon per year globally. This technique has gained some support as a way to enhance natural processes to sequester more atmospheric CO2 and potentially slow global warming.
However, ocean fertilization remains controversial. Dumping large amounts of iron or other nutrients into the oceans could have unintended consequences and disrupt marine ecosystems. More research is still needed to determine if this approach is feasibly and safely able to remove enough carbon at a global scale to meaningfully impact climate change. But enhancing natural carbon sequestration processes through ocean fertilization does show some promise as one way humans could actively intervene to recycle additional carbon if applied responsibly.
Carbon Taxes
One method of encouraging carbon sequestration and discouraging carbon emissions is through the use of carbon taxes. Carbon taxes put a price on carbon by charging a fee to fossil fuel companies, manufacturers, and other entities based on the amount of carbon dioxide and other greenhouse gases they emit.
The idea is that putting a price on carbon emissions will encourage companies and consumers to move towards lower carbon alternatives and more energy efficient processes. The increased cost of using fossil fuels means renewable energy like solar and wind becomes more cost competitive. Companies are incentivized to adopt greener technologies and develop innovations to reduce emissions in order to avoid the carbon tax.
Carbon taxes also generate revenue that governments can use to invest in renewable energy infrastructure, public transportation, environmental restoration, and assisting communities impacted by climate change. Instead of viewing the atmosphere as a free waste dump for greenhouse gases, carbon pricing reflects the real societal cost of emissions.
By making pollution more expensive, carbon taxes help shift the economy towards sustainability and clean energy while curbing the greenhouse gas emissions that drive climate change. It uses market forces to encourage emission reductions in a flexible and cost-effective way.