What Is Thermal Energy Transferred From A Region Of?
What is Thermal Energy?
Thermal energy refers to the total kinetic and potential energy of atoms and molecules in a substance. It is often referred to as heat energy and is associated with the movement of atoms or molecules within a substance. As an object gets warmer, its atoms and molecules move and vibrate faster, increasing the thermal energy.
Thermal energy flows spontaneously from objects at higher temperatures to objects at lower temperatures. For example, when you hold an ice cube in your hand, thermal energy flows from your hand (higher temperature) to the ice cube (lower temperature), causing the ice to melt. This process continues until both objects reach the same temperature.
Some common examples of thermal energy transfer include:
- Heating or cooling of objects
- Boiling of water into steam
- Condensation of steam into water
- Melting of ice into liquid water
- Freezing of water into ice
Methods of Thermal Energy Transfer
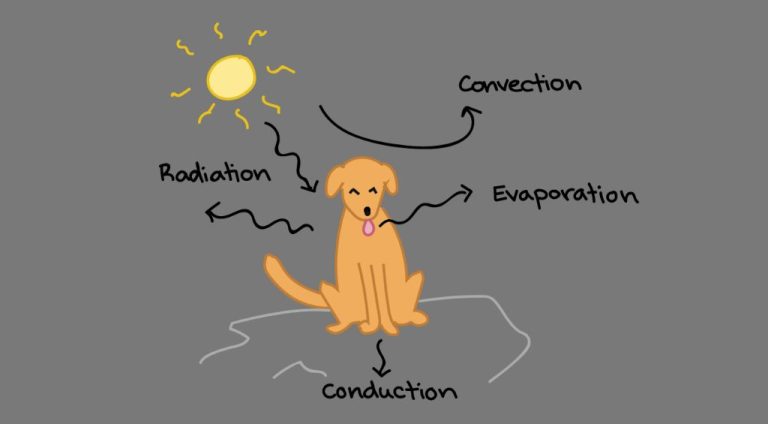
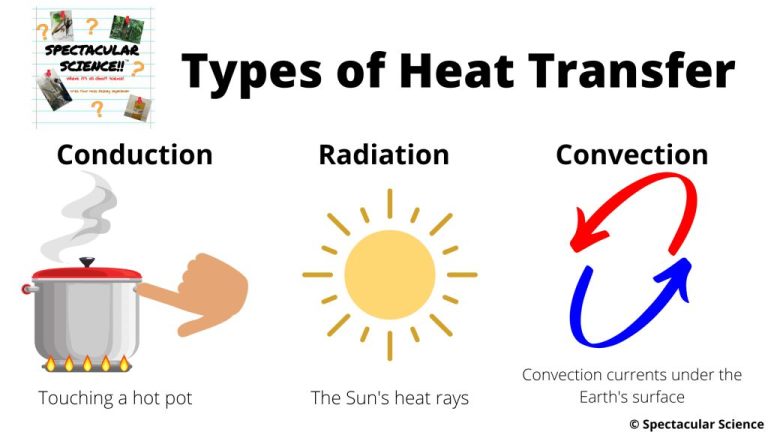
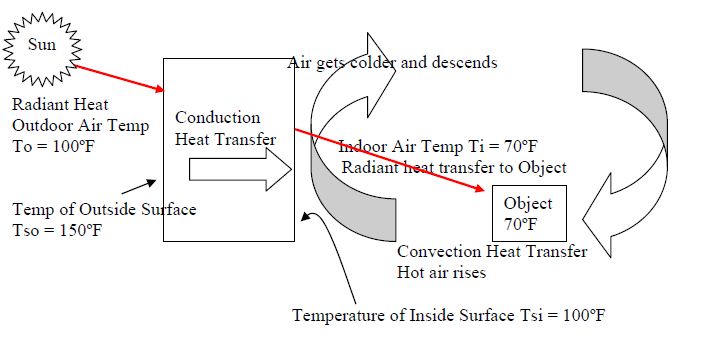
There are three main methods by which thermal energy is transferred from one object or system to another: conduction, convection, and radiation.
Conduction is the transfer of thermal energy between objects in direct physical contact with each other. The better the conducting material, the faster thermal energy will flow through it. Metals like copper and aluminum are excellent conductors. An example of conduction is a metal spoon getting hot after being left in a hot cup of soup.
Convection is the transfer of thermal energy by the movement of liquids and gases. As the fluid is heated, it expands, becomes less dense, and rises. Cooler, denser fluid then moves in to take its place, setting up a circulation pattern. This allows heat to be transferred away from a heat source. Examples of convection include warm air rising from a radiator or the churning motion of boiling water.
Radiation is the transfer of thermal energy by electromagnetic waves. All objects emit some level of thermal radiation related to their temperature. Thermal radiation does not require particles for energy transfer. An example is the warmth felt on your skin from the sun’s rays.
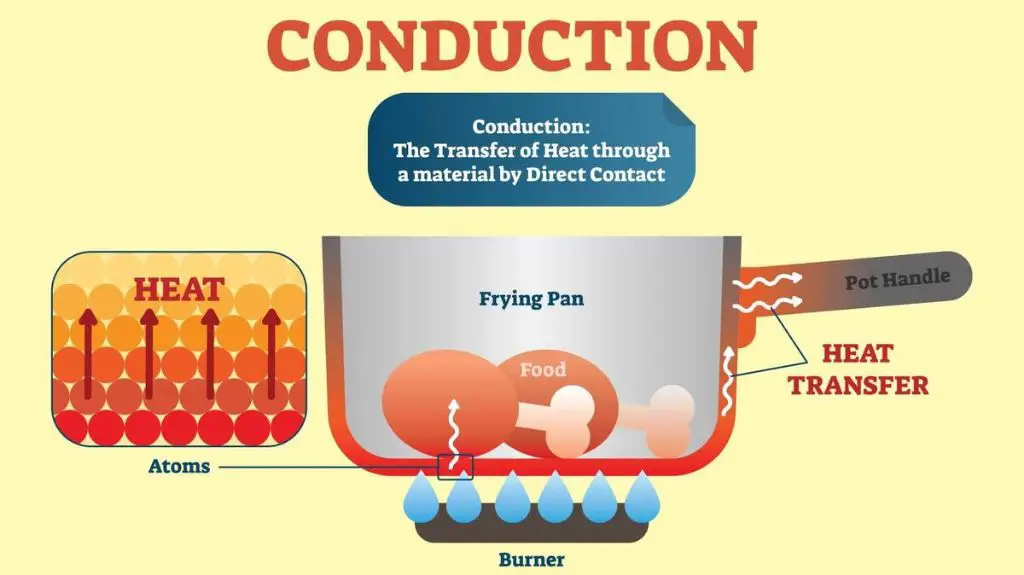
Conduction
Conduction is the transfer of thermal energy between objects that are in direct physical contact with each other. It occurs when heat flows from a region of higher temperature to a region of lower temperature. The rate of conductive heat transfer depends on the temperature difference between the regions as well as the thermal conductivity of the materials involved.
Conduction can occur in solids, liquids and gases. In solids, it is the vibration and diffusion of molecules that allows thermal energy to be transferred between neighboring particles. Metals tend to be good conductors as their freely moving electrons can quickly transfer thermal energy. Insulators have lower rates of conduction because of the weaker interactions and motion of their particles and electrons.
In liquids and gases, conduction occurs as particles collide and transfer kinetic energy. Heat conduction in gases is more complex though, as additional energy transfer can occur through diffusion and convection.
Examples of heat conduction include a spoon warming up when placed in a hot cup of soup. The heat transfers from the high temperature soup to the cooler spoon through direct contact. Another example is heat flowing downwards through the solid materials in a hot cooking pan. Conduction constantly transports thermal energy from hotter to cooler areas until equilibrium is reached.
Convection
Convection is the transfer of thermal energy via the movement of fluids, such as air or water. When a fluid is heated, the molecules begin to move faster and spread apart, decreasing the density of the fluid. The warmer, less dense fluid will then rise up, while the cooler, denser fluid will sink down. This movement and circulation allows for the transfer of heat.
Convection occurs in liquids and gases, or fluids. It does not happen in solids, since the molecules in a solid are not free to move around in the same way. Some examples of convection include boiling water, the motion of air during storms, and the currents that form in Earth’s oceans and atmosphere. As the fluid circulates and transfers heat, convection helps distribute thermal energy from hotter to cooler areas, eventually reaching an equilibrium.
Convection plays a major role in weather patterns, ocean currents, and the transfer of heat across Earth’s surface. It is also utilized in many heating and cooling systems, from the circulation of refrigerant in air conditioners to the heating coils and fans in convection ovens.
Radiation
Radiation is the transfer of thermal energy via electromagnetic waves, also known as photons. Unlike conduction and convection, radiation does not require a medium for the energy to travel through. Thermal radiation is emitted by all objects above absolute zero based on their temperatures. Hotter objects emit more thermal radiation than cooler objects.
A common example of thermal radiation is the energy emitted by the Sun. Although the Sun’s core reaches extreme temperatures of over 15 million degrees Celsius, the Sun’s surface temperature is about 5,500 degrees Celsius. At this temperature, the Sun emits energy in the form of ultraviolet, visible light, and infrared radiation. This solar radiation travels through the vacuum of space until it reaches Earth’s atmosphere and surface, transferring thermal energy in the process.
Infrared radiation is also emitted by objects at much lower temperatures, such as the human body and Earth’s surface. Radiative heat transfer plays an important role in maintaining Earth’s energy balance and regulating global temperatures.
Examples of Thermal Energy Transfer
Thermal energy transfer has many common examples in everyday life.
Heating and Cooling of Buildings
Buildings rely on heating and cooling systems to regulate indoor temperatures. Furnaces and boilers use the transfer of thermal energy to heat buildings, often transferring heat from burning fuel into air or water which is circulated through the rooms. Air conditioners and heat pumps transfer thermal energy out of indoor spaces to cool them off.
Cooking Food
Cooking appliances like stovetops, ovens, and microwaves all rely on thermal energy transfer to heat up food. The high temperatures denature proteins, caramelize sugars, and kill microorganisms in food through conduction, changing the chemistry and taste.
Car Engines
Combustion engines in cars and trucks generate thermal energy from exploding fuel and air mixtures. This thermal energy is transferred to move pistons, powering the vehicle. Additional systems also use thermal energy transfer to warm up or cool down the engine and heat or air condition the vehicle interior.
Earth’s Climate System
On a global scale, thermal energy is constantly being transferred between the atmosphere, oceans and land. Incoming solar radiation warms the tropics more than polar regions, setting up temperature differences that drive convection currents in air and water, distributing heat energy around the planet through weather and ocean currents.
Measuring Thermal Energy Transfer
In order to measure thermal energy transfers, we rely on specialized instruments and standardized units of measurement. Two key instruments used are:
- Thermometers: A device used for measuring the temperature of a system. Temperature reflects the average kinetic energy of atoms and molecules; higher temperatures correlate to greater atomic motion. Thermometers allow us to quantitatively track thermal energy changes. Common scales include Celsius, Fahrenheit and Kelvin.
- Calorimeters: An enclosed chamber used to measure the heat absorbed or released by a system. By measuring parameters like the temperature change in the calorimeter, one can precisely calculate the amount of thermal energy transferred in units of joules or calories.
Key units used to measure thermal energy changes include:
- Degrees (°C, °F, K): Temperature scales quantify the average kinetic energy of molecules in a system. Tracking degrees over time charts the net thermal energy gained or lost.
- Joules: The joule is the standard international unit used to quantify thermal energy, defined as the work done when a force of one newton displaces an object one meter. The calorie is also occasionally used.
Using thermometers, calorimeters and standardized units allows precise measurement of conduction, convection and radiation between systems.
Applications and Impact
Thermal energy transfer has many practical applications and a significant impact on energy usage and production. Some key examples include:
Heating and Cooling Technologies
Many technologies harness thermal energy transfer to heat or cool indoor environments. Heating systems like furnaces and boilers rely on conduction and convection to transfer heat from a heat source into the surrounding air. Air conditioners and refrigerators transfer heat out of the indoor space using a refrigeration cycle.
Insulators to Reduce Transfer
Materials like fiberglass, foam, wool, and certain plastics are designed to limit thermal energy transfer through conduction and convection. They are often used as insulation in walls, attics, pipes, and other locations to reduce heat gain or loss and therefore reduce energy consumption for heating and cooling.
Geothermal Energy Production
The natural thermal energy stored beneath Earth’s surface can be harnessed for geothermal power production. Hot subsurface rocks transfer heat to a working fluid that flashes into steam to spin turbines and generate electricity without combustion or emissions.
Thermal Energy Transfer in Earth’s Systems
The Earth’s atmosphere, oceans, and land receive, transmit, and dissipate large amounts of thermal energy, which drives weather and climate patterns.
The atmosphere, largely warmed through solar energy absorption, transfers thermal energy around the planet through cycles of evaporation, cloud formation, and precipitation. The movement of energy from warm sea areas to cooler areas through offshore and onshore winds transfers energy from the equatorial regions and oceans to land, driving surface weather and ocean currents.
The thermal energy stored in the oceans is enormous and the force driving the global ocean conveyor belt system that promotes mixing. Heat absorbed through the sun’s radiation by the oceans is largely released to the atmosphere, particularly in the tropics. Oceans interact with inland areas as well, transferring energy through evaporation, storms, and coastal winds.
The land absorbs and transfers thermal energy from both the atmosphere and oceans. When the land is warmed faster than the oceans, energy drives wind and ocean currents from the land back towards the sea. Transfers of thermal energy into and out of the land surface, soil, and atmosphere interface cause warming and cooling of inland areas on a daily and seasonal basis.
Conclusions
Thermal energy transfer refers to the movement of heat from one place to another through conduction, convection, or radiation. Conduction occurs through direct contact, convection occurs through motion of fluids, and radiation occurs through electromagnetic waves. Understanding the mechanisms of thermal energy transfer is important in science and engineering for analyzing and controlling heat flow in systems.
As discussed, thermal energy transfer impacts many natural processes in earth and life sciences as well as technological systems like heating, cooling, and power generation. Additional examples were provided of thermal conduction in cooking, convection in weather systems, and radiation from the sun. We also covered methods for quantifying and measuring thermal energy transfer such as thermocouples and calorimeters.
In summary, thermal energy refers to molecular kinetic energy, while heat flow describes the transfer of that energy between objects or locations. A grasp of thermal transfer principles helps explain observations of the natural world and allows purposeful applications in designed systems. This content aimed at clearly communicating key thermal transfer concepts for readers to gain important scientific knowledge on the topic.