What Is Thermal Energy Of A System Apex?
Thermal energy refers to the total internal energy present in a system due to the motion of its molecules and atoms. It arises from the kinetic energy of random motion of particles that make up matter. The faster the molecular motion, the higher the thermal energy. Thermal energy is a fundamental thermodynamic property that determines the temperature of matter. It provides a measure of the total energy contained within a system that can be converted into mechanical work.
The concept of thermal energy is central to thermodynamics, the branch of physics concerned with heat and temperature. It helps explain phenomena like heat transfer, thermodynamic processes, and phase transitions. An understanding of thermal energy is crucial for analyzing the performance of heat engines, refrigerators, and other thermal devices. It also aids in interpreting chemical bonding and material properties from a microscopic perspective.
In summary, thermal energy is a key variable for characterizing the thermodynamic state of systems. It offers deep insight into the behavior of matter and underlies many essential technologies we use today. A grasp of thermal energy is imperative for anyone working in physics, chemistry, engineering, and related fields.
Internal Energy
Internal energy is the total kinetic and potential energy of all the molecules within a thermodynamic system. It arises from the motions and interactions between molecules, including both kinetic energy from molecular motion as well as potential energy from molecular bonds and configurations. Internal energy is an extensive property, meaning it depends on the amount of material in the system.
Internal energy is directly related to the thermal energy of a system. Thermal energy refers to the total internal kinetic and potential energy associated with the microscopic motions and configurations of molecules and atoms. As the internal energy of a system increases, so does its thermal energy since they are intrinsically linked. This is why heating an object, which adds internal energy, also increases its temperature – a reflection of thermal energy.
In summary, internal energy is the sum total of all forms of energy at the molecular level in a thermodynamic system. Changes in internal energy are associated with changes in thermal energy, so understanding internal energy provides insights into the thermal properties of systems.
Heat
Heat is a form of energy transfer between objects or systems due to a temperature difference. When two objects at different temperatures come into contact, heat flows spontaneously from the hotter object to the colder one until they reach thermal equilibrium. Heat always flows from higher temperature to lower temperature.
Heat is different from thermal energy. Thermal energy refers to the total kinetic and potential energy of all the molecules within an object. This is an intrinsic property of matter that depends on the temperature, mass, and chemical composition. Heat, on the other hand, is energy in transit and not a property of a system. Adding heat to an object increases its thermal energy.
The amount of heat transfer is directly proportional to the temperature difference between the objects. The greater the temperature difference, the faster the heat transfer rate. Heat transfer stops once thermal equilibrium is reached and the objects are at the same temperature. Measuring the amount of heat transfer gives information about the thermal energy state of a system.
Temperature
Temperature is a measure of the average kinetic energy of the molecules or atoms in a substance. It refers to how hot or cold an object is. Temperature is directly proportional to the thermal energy of a system. As thermal energy increases, temperature also increases. This is because increased thermal energy means the molecules and atoms are moving faster and have more kinetic energy.
Temperature helps quantify thermal energy. While thermal energy refers to the total kinetic and potential energy of all the molecules in a system, temperature offers a measure of just the average kinetic energy. Tracking changes in temperature allows us to understand how the thermal energy changes as well. For example, as you heat up water on a stove, adding thermal energy increases the average kinetic energy of the water molecules, which is observed as a rise in temperature.
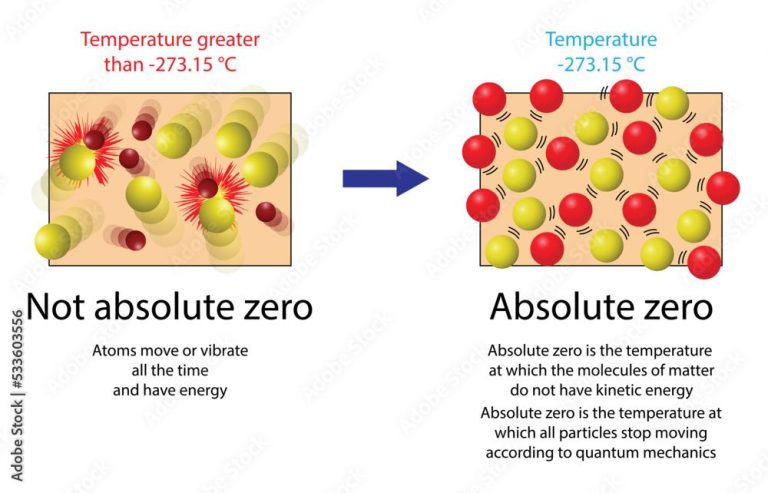
There are various temperature scales used to measure this kinetic energy precisely. Common scales include Fahrenheit, Celsius, and Kelvin. The Kelvin scale is the standard unit of temperature in the International System of Units (SI). Scientists often use the Kelvin scale because it starts at absolute zero, the temperature at which molecules have no kinetic energy and don’t move. The other temperature scales like Celsius and Fahrenheit have arbitrary zero points based on the freezing point of water.
Overall, temperature provides a convenient way to track thermal energy changes in a system. The two concepts are directly related – increasing the thermal energy leads to a rise in temperature, while decreasing thermal energy leads to a drop in temperature. By monitoring temperature changes, we can understand how the kinetic energy of molecules and atoms evolves as energy is added or removed from a system.
Entropy
Entropy is a scientific concept that is closely tied to thermal energy and thermodynamics. Entropy is a measure of the disorder of a system. The higher the entropy, the more disordered a system is. Entropy broadly refers to the number of specific ways the constituents of a system can be arranged. The more arrangements available to a system, the higher its entropy.
Entropy is directly connected to thermal energy and heat. When heat flows into a system, it increases the number of possible arrangements of the components in that system, thus increasing entropy. This is because the energy from heat provides more kinetic energy to the molecules and atoms that make up the system, allowing them to move and vibrate more. The more ways these particles can arrange themselves, the higher the entropy.
Adding heat to a system spreads energy around and makes energy less concentrated. This increase in disorder is reflected as an increase in entropy. Removing heat decreases a system’s entropy. When a system loses thermal energy, motion decreases, fewer arrangements of particles become accessible, and entropy goes down.
In summary, entropy is a measure of disorder that is directly connected to the thermal energy, kinetic energy, and heat energy present in a system. The more heat is added to a system, the higher its entropy becomes. Entropy provides deep insight into the behavior of thermal energy and heat transfer at the microscopic level.
Measuring Thermal Energy
There are several instruments and techniques used to measure thermal energy in physics and engineering applications.
Thermometers are the most common instruments used to measure temperature, which is directly related to thermal energy. Various types of thermometers include mercury thermometers, alcohol thermometers, infrared thermometers, thermocouples, and resistance temperature detectors (RTDs). Each type works on different principles and has its own temperature measurement range and accuracy.
Calorimeters allow measuring the amount of heat absorbed or released during a chemical or physical process. Simple calorimeters like bomb calorimeters directly measure temperature change, while more complex devices like differential scanning calorimeters can identify phase transitions.
Thermal imaging cameras can visualize and quantify infrared radiation emitted by objects, creating images of surface temperature. They are useful for applications like building insulation efficiency, mechanical system monitoring, and medical imaging.
Additional techniques include using thermographic phosphors, thermometric titrations, and scanning thermal microscopy. The choice of technique depends on the temperature range, system under study, and quantitative vs qualitative measurement needs.
Computer modeling and simulation is another important approach. Software tools can model heat transfer mechanisms and provide thermal data for systems in cases where real measurements are difficult.
Understanding these techniques is key for studying and applying knowledge of thermal physics across science and engineering fields.
Thermal Energy Transfer
Thermal energy can be transferred between objects or systems through three main mechanisms: conduction, convection, and radiation.
Conduction is the transfer of heat between objects that are in direct physical contact with each other. It occurs when faster moving atoms or molecules interact and transfer some of their energy to slower moving neighboring atoms or molecules. Metals are good conductors of thermal energy. Conduction allows thermal energy to spread from hotter to cooler regions to eventually reach thermal equilibrium.
Convection is the transfer of heat by the actual movement of hot or warm matter. It occurs in liquids and gases, where hot regions become less dense than cool regions, causing them to rise while cool regions sink. This sets up convection currents that help transfer heat. Convection allows for widespread, large-scale transfers of thermal energy in fluids. Examples include heating or cooling a room with air currents, and the large-scale motion of ocean currents and molten lava.
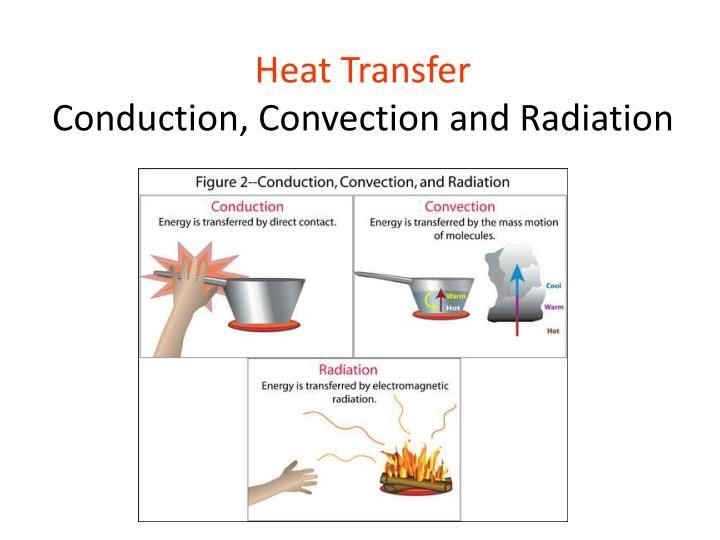
Radiation is the transfer of heat via electromagnetic waves or photons. No physical contact is required. All objects emit infrared radiation depending on their temperature. The net transfer of radiant energy is from the hotter to the cooler object. Radiation is the only method of heat transfer possible in a vacuum, such as space. The sun transfers thermal energy to Earth mainly through radiation.
Thermal Energy in Physics
Thermal energy plays an important role in physics, particularly in the branch of physics known as thermodynamics. Thermodynamics deals with heat and temperature and their relation to energy, work, radiation, and properties of matter. Some key concepts involving thermal energy in physics include:
The Laws of Thermodynamics – The laws of thermodynamics describe the relationships between thermal energy, heat, work and the property of systems. The First Law states that energy is conserved. The Second Law states that the entropy of an isolated system always increases. The Third Law defines absolute zero on the thermodynamic temperature scale.
Heat Transfer – Heat transfers from high temperature to low temperature through conduction, convection and radiation. The rate of conductive heat transfer depends on the temperature difference and the thermal conductivity of materials. Convection involves the bulk movement of fluids driven by temperature differences. Radiation occurs when objects emit electromagnetic waves due to their temperature.
Thermodynamics Engines – Heat engines like car engines, steam turbines and refrigerators utilize thermal energy transfers to do work. Their efficiency is determined by thermodynamic cycles that transform heat into work via processes like isothermal expansion and adiabatic compression.
Phase Transitions – Phase transitions like melting, boiling, condensation and freezing involve large transfers of latent heat at constant temperature. These phase changes are governed by thermodynamic laws and properties like internal energy and entropy.
Kinetic Theory – The kinetic theory describes gases as large numbers of sub-microscopic particles in random motion. It links the temperature of gases to the average kinetic energy of the particles and leads to relations between pressure, volume and temperature.
Thermal Energy in Chemistry
Thermal energy concepts play an important role in chemistry, particularly when looking at chemical reactions. One of the most fundamental ideas is the distinction between exothermic and endothermic reactions.
Exothermic reactions are those that release energy in the form of heat. Examples include combustion reactions, like the burning of fossil fuels, and reactions between acids and bases. These reactions give off heat to the surroundings and have a negative change in enthalpy.
In contrast, endothermic reactions absorb heat energy from the surroundings. Examples include photosynthesis in plants and the reaction between baking soda and vinegar. These require an input of energy to proceed and have a positive change in enthalpy.
The transfer of thermal energy during chemical reactions can be measured using calorimetry. This allows chemists to determine the energy changes during reactions and gain insight into reaction mechanisms and chemical bonding.
Thermochemistry looks closely at the relationship between heat and chemical reactions. By studying the thermal energy changes, chemists can better understand reaction kinetics, equilibrium, and thermodynamics.
Applications of Thermal Energy
Thermal energy principles have many important real-world applications, especially in engineering and technology.
In thermodynamics, thermal energy concepts help engineers design more efficient engines, motors, and power plants. For example, understanding heat transfer and the laws of thermodynamics allows engineers to maximize the efficiency of internal combustion engines in automobiles and aircraft.
Thermal energy is also critical in the design of heating and cooling systems for buildings and electronics. HVAC engineers rely on knowledge of thermal conduction, convection, radiation, and heat exchangers to create climate control systems. Electrical engineers apply thermal principles to design heat sinks and cooling fans to regulate device temperatures.
Energy companies harness geothermal energy by extracting heat from underground hydrothermal resources. They use thermal energy concepts to drill wells, generate steam, and run turbines to produce renewable power. Geothermal energy has expanded as engineers apply thermal principles to extract heat from various sources like deep aquifers.
Manufacturers and industrial plants use thermal processes like smelting, annealing, tempering, and heat treatment to alter material properties. Careful control of thermal energy allows metallurgists to optimize material strength, hardness, ductility, and other characteristics.
Thermal energy even enables key medical applications like cryosurgery, thermal ablation, and magnetic hyperthermia cancer treatment. Overall, the broad relevance of thermal physics underlies many critical technologies and systems worldwide.