What Is The Process Where Energy Is Released?
Energy release, or energy liberation, is the process by which energy is transferred or transformed into a usable or accessible form. This occurs through various chemical or physical reactions and processes that result in a release of energy previously stored in the molecular bonds of a chemical compound or in the nucleus of an atom.
The most common example of energy release occurs during chemical reactions, where energy stored in the bonds between atoms is released as heat. This exothermic process powers everything from human metabolism to combustion engines. Nuclear reactions also result in tremendous energy release, as matter is converted to energy according to Einstein’s famous equation E=mc^2.
Understanding the mechanisms of energy release is key to harnessing this energy for constructive purposes, from generating electricity to powering transportation. Whether the goal is generating heat, light, motion, or electricity, the ability to control energy release in a safe, efficient manner has been central to technological innovation throughout human history. As energy needs continue to grow globally, optimizing energy release processes remains crucial for economic and environmental sustainability.
Activation Energy
Activation energy is the minimum amount of energy that is needed for a chemical reaction to take place. It is like the initial spark plug that gets the reaction going. Reactions will not occur unless molecules collide with each other with enough energy to overcome this activation barrier.
Activation energy is required to break bonds between atoms in the reactant molecules. This allows the atoms to rearrange and form new bonds to create the product molecules. Strong chemical bonds require high activation energy to break, while weak bonds have lower activation energy requirements.
Here are some examples to illustrate the concept of activation energy:
- The reaction between hydrogen and oxygen to form water requires a small flame or spark to initiate. This provides the activation energy to break the bonds in H2 and O2.
- ATP hydrolysis to release energy for cellular processes – The phosphate bonds in ATP must be “activated” by enzymes that lower the activation barrier.
- Food cooking with heat – Heat provides the activation energy for complex chemical changes like Maillard reactions.
In summary, activation energy is a critical concept for understanding reaction kinetics. Reactions will stall without enough activation energy to get over the initial barrier.
Exothermic Reactions
Exothermic reactions are chemical reactions that release energy in the form of heat and light. During an exothermic reaction, energy is transferred from the chemical bonds in the reactants to the surroundings. This results in the products having less energy than the reactants.
Some examples of exothermic reactions include:
- Combustion reactions – Common exothermic combustion reactions include burning fossil fuels like coal, oil, and natural gas, as well as the burning of wood. These reactions give off heat and light energy.
- Thermite reactions – Thermite is a mixture of aluminum powder and metal oxide. When ignited, thermite undergoes an extremely exothermic oxidation-reduction reaction, reaching temperatures over 2500°C.
- Acid-base neutralization – When an acid and base react and neutralize each other, salt and water are formed. This reaction is mildly exothermic.
The energy released during an exothermic reaction comes from the chemical bonds within the reactants. Bonds between atoms contain energy that can be released when those bonds are broken. In an exothermic reaction, the energy released when new bonds form in the products is less than the energy required to break the reactant bonds. This net release of energy is given off as heat.
Endothermic Reactions
An endothermic reaction is a chemical reaction that absorbs energy from its surroundings. This energy absorption leads to an increase in the energy of the reactants and a decrease in the temperature of the surroundings.
Some examples of endothermic reactions include:
- The thermal decomposition of ammonium dichromate into chromium(III) oxide, nitrogen gas, and water vapor:
- 2(NH4)2Cr2O7(s) → Cr2O3(s) + N2(g) + 4H2O(g) + Heat
- Photosynthesis in plants absorbs light energy from the sun to convert carbon dioxide and water into glucose and oxygen:
- 6CO2 + 6H2O + Light → C6H12O6 + 6O2
- The hydration of anhydrous copper(II) sulfate into blue copper sulfate crystals:
- CuSO4(s) + 5H2O(l) → CuSO4•5H2O(s) + Heat
During an endothermic reaction, energy is absorbed when bonds are broken in the reactants. This absorbed energy allows new bonds to form in the products, resulting in an overall increase in the internal energy of the system. The absorption of heat from the surroundings causes the temperature of the surroundings to decrease.
Chemical Bonds
Chemical bonds are what hold atoms together in molecules or crystals. Atoms form bonds to be more stable, but these bonds can also break. The formation and breaking of chemical bonds involves energy transfers that can release or absorb heat.
When a chemical bond forms between atoms, energy is absorbed. This is because the bonded atoms are at a lower energy level compared to when they were separate atoms. The absorbed energy is known as the binding energy. For example, when two hydrogen atoms bond to form a hydrogen molecule (H2), a certain amount of energy is absorbed in the process.
The breaking of chemical bonds requires energy input. Energy must be supplied to break the attractions between the bonded atoms. The energy needed to break a bond is equal to the binding energy. When bonds break, the energy that was initially absorbed in forming the bonds is released. This released energy can be in the form of heat.
The formation and breaking of chemical bonds during chemical reactions releases or absorbs energy. Exothermic reactions involve net breaking of bonds, resulting in heat released. Endothermic reactions involve net bond formation, absorbing heat. The energy changes associated with bond making and breaking drive the processes where energy is released.
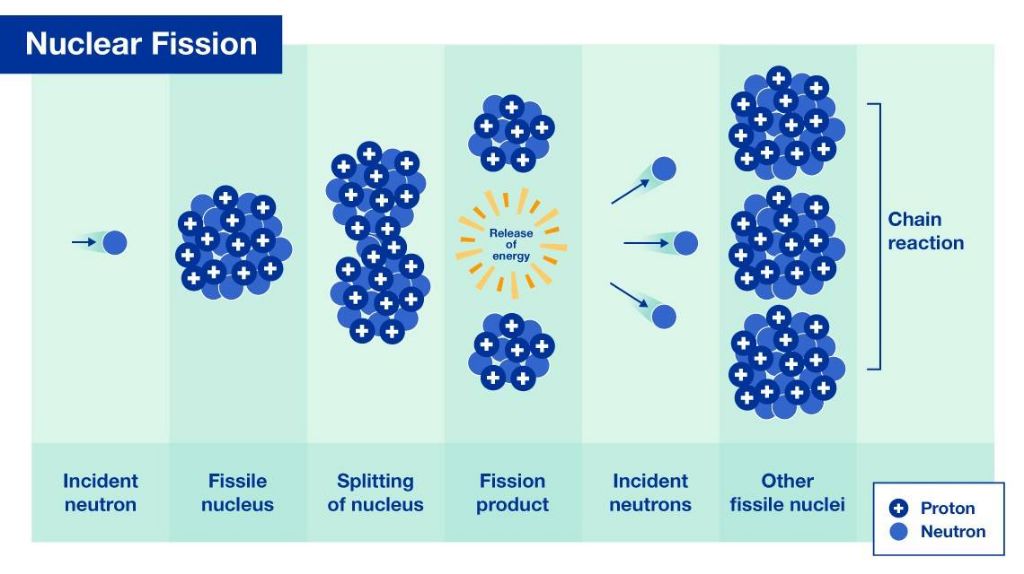
Nuclear Fission
Nuclear fission is the process whereby the nucleus of an atom splits into smaller parts. This usually happens when a neutron strikes the nucleus of a heavy element like uranium or plutonium. When the neutron hits the nucleus, it causes the nucleus to become unstable and split apart. As the nucleus splits, a tremendous amount of energy is released in the form of heat and radiation.
The process of nuclear fission also releases more neutrons which can cause other atoms to split, creating a chain reaction. In nuclear power plants, the chain reaction is controlled and sustained to produce a steady supply of energy. In nuclear weapons, the chain reaction is uncontrolled, causing an explosive release of energy.
Nuclear fission releases nearly a million times more energy than chemical reactions like burning fossil fuels. That’s why nuclear power plants need only a small amount of fuel to produce massive amounts of electricity. The intense energy release is also why nuclear weapons pack such a devastating punch.
Besides generating power, nuclear fission has applications in medical devices, space exploration, and national security. However, nuclear fission also carries risks if not properly contained and controlled. The resultant radiation can be hazardous without proper safeguards. Nonetheless, nuclear fission remains a tremendously energy-dense process to harness for humanity’s needs.
Nuclear Fusion
Nuclear fusion is the process by which multiple atomic nuclei join together to form a heavier nucleus, releasing enormous amounts of energy in the process. This is the same process that powers stars like our sun. For fusion to occur, tremendous heat and pressure are needed to force nuclei close enough together to overcome their natural repulsion. Temperatures over 100 million degrees Celsius are required to initiate and sustain fusion reactions.
The most promising fusion reaction for energy production is the fusion of two hydrogen isotopes, deuterium and tritium. These fuse together to form a helium nucleus, releasing a high-energy neutron in the process. The neutron carries the released binding energy kinetically. This kinetic energy can then be harvested to produce electricity.
Nuclear fusion holds tremendous promise as an energy source because it has several key advantages over current fission reactors. First, its fuel (hydrogen isotopes) is practically limitless. Second, it produces no greenhouse gases. And third, the radioactive byproducts have a much shorter half-life than fission waste products.
However, the extreme conditions needed for fusion have so far stymied efforts to build a net energy producing fusion reactor. Scientists are making progress though, with the largest experimental reactor called ITER set to begin operations within this decade. If successful, fusion power could provide a safe, clean, and virtually limitless source of energy to meet humanity’s needs in the 21st century and beyond.
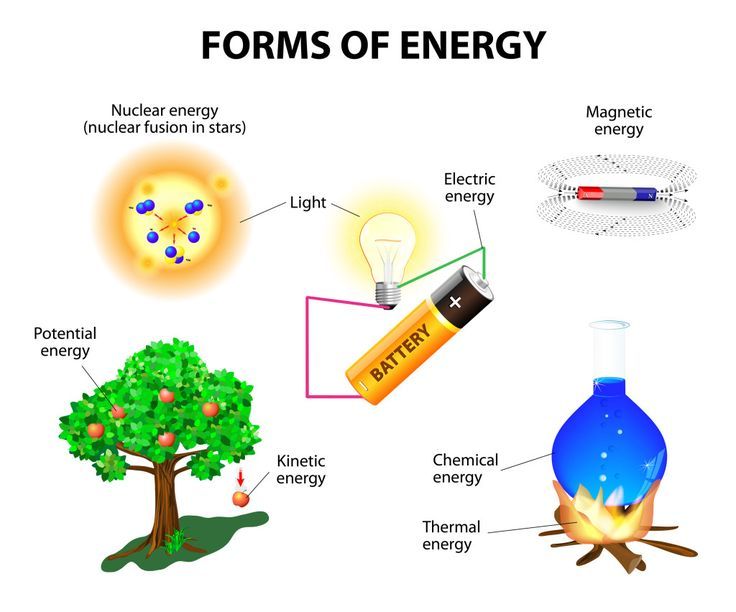
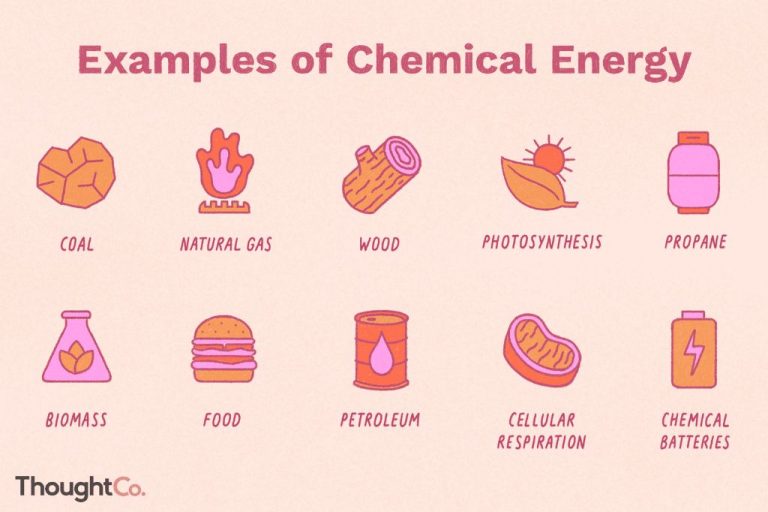
Chemical Energy
Chemical energy is the potential energy stored in the bonds between atoms and molecules. There are several ways that chemical energy can be released and converted into other forms of energy:
Stored Chemical Energy
Chemicals such as fossil fuels, biomass, and explosives contain stored chemical energy in their molecular bonds. When these bonds are broken through combustion, chemical decomposition, or detonation, the stored chemical energy is released usually as heat and light.
Batteries
Batteries convert chemical energy into electrical energy through redox reactions. The anode and cathode materials in the battery undergo oxidation and reduction reactions respectively when connected in a circuit, generating an electrical current.
Metabolism
Living organisms store chemical energy in organic molecules like carbohydrates, fats, and proteins. This energy is released through metabolic pathways that break down these molecules and convert their energy into forms usable by the cell, like ATP.
Thermodynamics
Thermodynamics is the branch of physics that deals with the relationships between heat, work, and energy. The laws of thermodynamics explain how energy can be transferred between systems in the form of heat or work. Thermodynamics plays a crucial role in understanding how energy is released in chemical and physical processes.
The first law of thermodynamics states that energy can neither be created nor destroyed in an isolated system. Energy can only be transferred from one form to another. This means that the total energy in the universe remains constant.
The second law of thermodynamics introduces the concept of entropy. Entropy is a measure of disorder in a system. The second law states that the entropy of an isolated system always increases over time. This tends to move energy from being concentrated in one place to being spread out and unavailable to do work.
These thermodynamic laws describe how energy flows between systems and set limits on how efficiently heat can be converted into useful work. Exothermic reactions involve a net release of energy, resulting in an increase in the entropy of the surroundings. Endothermic reactions require a net input of energy, so the entropy of the surroundings decreases. Understanding thermodynamics allows us to harness energy transfers to do useful work.
Conclusion
In summary, the process where energy is released is a complex topic with many facets. We have explored some of the key energy release processes like exothermic chemical reactions, nuclear fission and fusion, and chemical bonds breaking. It is important to have a foundational understanding of these energy release mechanisms as they power many essential systems in our world. From chemical processes in our bodies, to nuclear power production, to something as simple as burning wood for heat, we rely on these energy releases every day. Appreciating the physics and chemistry behind these reactions allows us to better harness them safely and efficiently. As our knowledge progresses, we may uncover new energy release processes that could further benefit society. But the core concepts of activation energy, bond breaking, fission, and fusion will continue to be relevant. Energy production and utilization is a key driver of civilization, and grasping the release of energy is crucial in this context.