What Is The Difference Between Thermal Energy And Heat?
Thermal energy and heat are related concepts in physics and thermodynamics. While they are often used interchangeably in everyday language, they have distinct scientific definitions. Thermal energy refers to the total internal energy of an object due to the random motion of its atoms and molecules. Heat, on the other hand, is the transfer of thermal energy between objects at different temperatures.
This article will examine the precise definitions of thermal energy and heat, explain the relationship between the two, and provide examples to illustrate the differences. We will also discuss how thermal energy and heat are measured, as well as applications like heat transfer and thermodynamics where distinguishing between the two concepts is essential.
Definitions
When discussing energy, it’s important to understand the differences between two related but distinct concepts – thermal energy and heat.
Thermal energy refers to the total internal kinetic energy of all the molecules within a substance. This internal energy consists of the random motions and vibrations of the atoms, molecules, and other particles that make up a substance. The faster these particles move, the more thermal energy the substance contains. Thermal energy is measured in joules and is an intrinsic property of matter that exists on a microscopic scale.
In contrast, heat is the transfer of thermal energy between substances due to temperature differences. It flows spontaneously from a substance at a higher temperature to a substance at a lower temperature. Heat itself is energy in transit and does not remain with the substance. Heat has the same units as thermal energy (joules) and can be measured by the temperature change it produces.
So in summary:
- Thermal energy is the total internal kinetic energy of the molecules in a substance.
- Heat is the transfer of thermal energy between substances.
Relationship Between Thermal Energy and Heat
Although the terms thermal energy and heat are sometimes used interchangeably, they refer to distinct but related concepts. Thermal energy is the total internal kinetic and potential energy of all the molecules within a substance. It is related to the temperature of the substance and the movements and vibrations of its molecules and atoms.
Heat, on the other hand, refers specifically to the transfer of thermal energy between substances or objects that are at different temperatures. When two objects with different temperatures come into contact, thermal energy will spontaneously transfer from the higher temperature object to the lower temperature object. This flow of thermal energy is heat.
So in summary, thermal energy is contained within a substance, while heat is the movement of thermal energy between substances. Heat is the transfer of thermal energy driven by temperature differences, and results in energy being lost by the hotter substance and gained by the colder substance.
Temperature vs Thermal Energy
Though related, temperature and thermal energy are distinct scientific concepts. Temperature measures how hot or cold an object is, while thermal energy refers to the total energy of microscopic motions of particles in a system.
Temperature is measured on a scale, such as Celsius or Fahrenheit. It describes the average kinetic energy of molecules within a substance. Higher temperatures mean the molecules have more kinetic energy and are moving faster on average.
Thermal energy, also called internal energy, is the total potential and kinetic energy associated with the random motions of atoms and molecules in an object. It depends on the number of atoms, their mass, and how vigorously they are moving. More thermal energy means more total microscopic motion within the molecules.
For example, a pot of boiling water and a swimming pool at the same temperature of 100°C have vastly different amounts of thermal energy. The swimming pool has far more water molecules, thus greater total kinetic energy, even though their average motions, as measured by temperature, are the same.
Understanding the distinction between temperature and thermal energy is key for fields like thermodynamics and engineering. While related, temperature measures molecular motion on average, while thermal energy quantifies the total kinetic energy from molecular motion within a system.
Measuring Thermal Energy
Thermal energy is measured in joules or calories. A joule is the SI unit of energy, while a calorie is based on the energy needed to raise one gram of water by one degree Celsius. Specifically, one calorie is the amount of thermal energy required to raise one gram of water from 14.5°C to 15.5°C under standard atmospheric pressure.
In the metric system, the joule is the standard unit used to measure energy. One joule (J) is defined as the work required to produce one watt of power for one second. For thermal energy, this translates to the amount of heat required to raise one gram of water by 0.24 degrees Celsius. The calorie and joule are convertible units, with 1 calorie equaling 4.184 joules.
To measure the thermal energy of an object or system, the total heat energy can be quantified in joules or calories. This is done by measuring the temperature change as energy is added or removed. The specific heat capacity of the material must also be known, which indicates how much energy is required to change its temperature by one degree. By calculating the mass, temperature change, and specific heat, the total thermal energy can be determined through the equation:
Thermal Energy = Mass x Specific Heat Capacity x Temperature Change
This allows the thermal energy contained within or transferred to/from an object or system to be numerically measured in joules or calories. This quantification is essential for thermodynamic analysis and calculations in science and engineering.
Measuring Heat
Heat is measured in units of energy. The two main units used to measure heat are joules and calories:
Joules – The joule is the SI unit of energy in the International System of Units. One joule is defined as the amount of work required to produce one watt of power for one second. Joules are commonly used by scientists to measure heat and energy in various contexts.
Calories – A calorie is defined as the amount of heat energy needed to raise the temperature of 1 gram of water by 1 degree Celsius. Calories are commonly used to measure the energy content in foods. Nutrition labels on food packages often list the calorie content per serving.
In science, joules are more commonly used than calories when measuring heat energy. However, in everyday contexts, calories are still frequently used, especially when discussing food energy content. For example, the amount of heat needed to boil a pot of water could be measured in joules, while the amount of energy contained in a candy bar would be measured in calories.
Heat Transfer
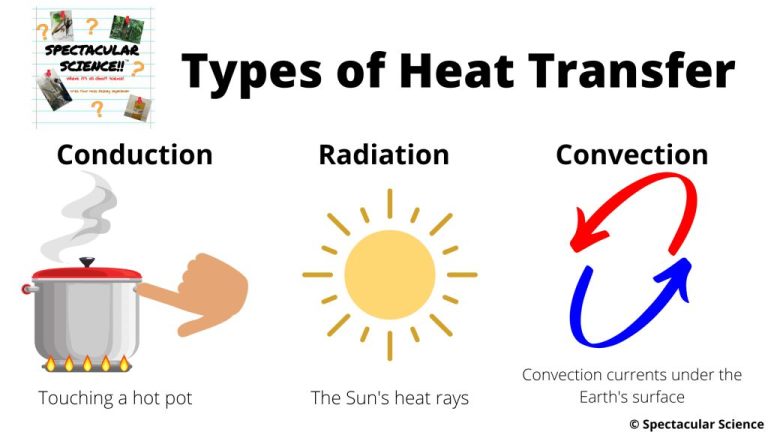
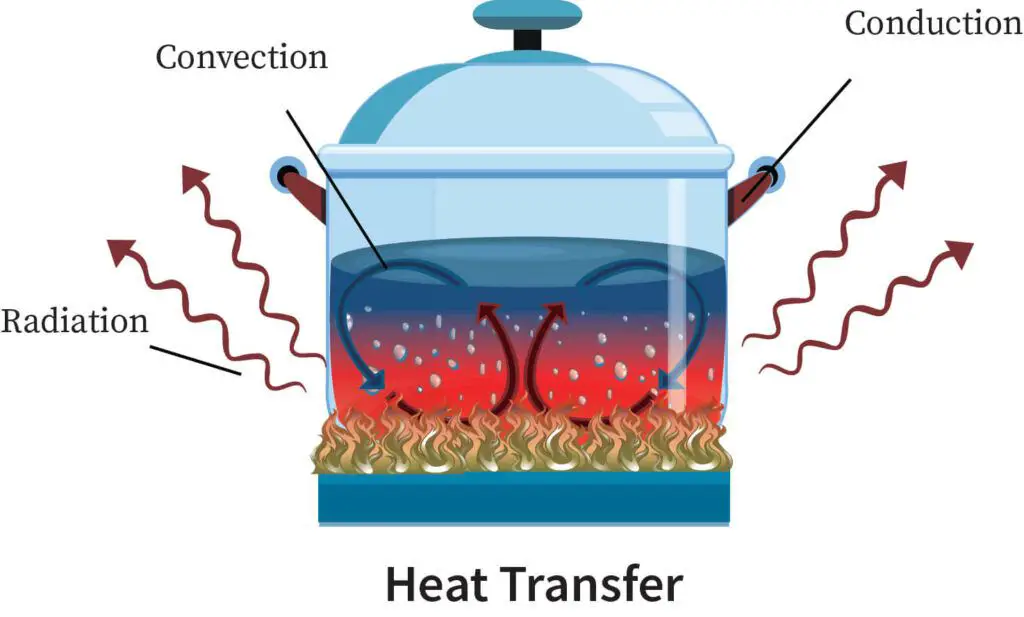
Heat can be transferred between objects or systems through three main methods: conduction, convection, and radiation.
Conduction is the transfer of heat between objects or systems that are in direct contact with each other. It occurs when atoms and molecules with higher kinetic energy transfer energy to neighboring atoms and molecules with lower energy. Metals are good conductors of heat because their atoms can easily vibrate against each other and pass kinetic energy.
Convection is the transfer of heat by the movement of heated fluid particles between objects or systems. It often involves an intermediary substance like air or water. For example, as air is heated it becomes less dense and rises, allowing cooler denser air to take its place, creating convection currents. This allows heat to be dispersed through the movement and mixing of the air.
Radiation is the transfer of heat via electromagnetic waves or photons. It does not rely on direct contact or any intermediary substance. For example, the sun radiates heat to the Earth across the vacuum of space. The heat we feel from a fireplace is also radiant heat transferred by infrared waves. Radiant heat transfer decreases with increasing distance, as radiation spreads out and loses intensity further away from the heat source.
Understanding these three heat transfer mechanisms is important for designing effective heating and cooling systems, predicting weather patterns, and much more.
Examples
Hot tubs illustrate the difference between thermal energy and heat well. The water in a hot tub contains thermal energy due to the motion of its molecules. The motors and heaters in the hot tub’s equipment provide the energy needed to increase the water’s thermal energy, raising its temperature. The thermal energy in the now hot water can transfer heat to people sitting in the hot tub. The people absorb the heat, feeling warmer as it transfers from the water to their bodies. However, the total thermal energy in the system (the hot tub water) does not change during this heat transfer.
Another example is a pot of water on the stove. Adding heat from the stove burner increases the water’s thermal energy, making the water molecules move faster. This adds heat to the water, raising its temperature. However, if the pot is not insulated, heat transfers from the hot water to the surrounding air. As heat leaves the water, the water’s temperature decreases, but its total thermal energy remains constant. This illustrates how heat can be gained or lost, while thermal energy remains fixed.
A final example is a glass of ice water with ice cubes floating in it. The glass contains thermal energy due to the motion of the water molecules. The thermal energy is lower than it would be without the ice, so the drink is cold. As heat transfers from the surrounding air into the drink, the ice absorbs it, keeping the drink cold. The ice acts as a heat sink, absorbing heat without changing its own thermal energy. It melts as it absorbs the heat entering the glass. This example shows how heat transfer impacts temperature, while thermal energy remains unchanged.
Practical Applications
Thermal energy and heat are central to many practical applications that power modern society, especially energy generation and engines.
One key example is the internal combustion engine, used in most cars and trucks. This engine burns gasoline, converting the chemical energy in the fuel into thermal energy through combustion. This thermal energy rapidly heats and expands the air in the engine’s cylinders, pushing the pistons and generating mechanical energy that powers the vehicle.
The principle behind most electric power generation also relies on thermal energy and heat. In coal, gas, or nuclear power plants, thermal energy released from burning fuel or nuclear reactions produces steam. This high-pressure steam then spins a turbine connected to an electrical generator, converting the thermal energy into electricity.
Solar thermal systems harness heat from the sun’s rays to warm water or spaces. The sunlight striking the solar panels contains thermal energy that gets transferred to a liquid, which can then heat buildings, swimming pools, or water for domestic use.
Understanding thermal energy flows and heat transfer is also crucial in designing effective heating and cooling systems, insulating buildings, and increasing energy efficiency in homes and industry.
Conclusion
In summary, thermal energy and heat are related but distinct concepts. Thermal energy refers to the total kinetic energy of molecules within a substance, which relates to the temperature of the substance. Heat refers specifically to the transfer of thermal energy between objects or systems due to a temperature difference. While thermal energy is contained within a system, heat is energy in transit. Measuring thermal energy requires complex calculations using thermodynamic properties. Heat is easier to measure based on temperature change and the heat capacity of a system. Understanding the nuanced difference between thermal and heat is key for applications like designing heat exchangers, solar panels, and other thermodynamic systems.