What Is The Difference Between Heat And Energy In Thermodynamics?
Thermodynamics is a branch of physics that deals with heat, energy, and work and their relations. Understanding the differences between heat and energy is foundational to thermodynamics. As we will see, heat and energy are two distinct concepts that behave differently and must be analyzed using different laws and principles.
Heat is the transfer of thermal energy between systems due to temperature differences. It is a process that changes the internal energy of a system by adding or subtracting energy. Energy is the capacity to do work. It exists in various forms like kinetic, potential, chemical, thermal, electrical, etc. Energy is always conserved, meaning it cannot be created or destroyed, only transformed from one form to another.
Grasping the nuances between heat and energy is crucial for analyzing thermodynamic processes. How heat flows, how it converts between different energy types, and the efficiency of energy conversion all rely on properly distinguishing heat from energy. Mastering these concepts allows us to improve engineering systems, meet energy demands, and further scientific knowledge.
Define Heat
Heat is the flow of thermal energy between systems due to their temperature difference. It refers specifically to the amount of thermal energy that is being transferred between systems, from the system with a higher temperature to the one with a lower temperature. Thermal energy itself is the kinetic energy of the molecules or atoms that make up a system due to their random thermal motions and vibrations.
Heat always flows spontaneously from a body at a higher temperature to one at a lower temperature. This transfer of thermal energy continues until the two systems reach the same temperature, known as thermal equilibrium. At this point, the thermal energy is distributed evenly between the systems and no further net heat transfer occurs.
While thermal energy is an extensive property of matter that depends on the amount of material, heat is an intensive property, defined only by the temperature difference and the path of transfer. Heat flow does not change the quantity of thermal energy present, it simply redistributes it between objects or locations.
Define Energy
Energy is the ability to do work or produce heat. It exists in various forms such as kinetic, potential, thermal, chemical, nuclear and more. Energy can be transferred or transformed from one form to another, but it cannot be created or destroyed according to the law of conservation of energy.
There are two main types of energy – potential energy and kinetic energy. Potential energy is stored energy that an object has due to its position or chemical composition. For example, a ball held at a height above the ground has gravitational potential energy due to earth’s gravity acting on it. Kinetic energy is energy of motion that a moving object possesses. For example, a rolling ball has kinetic energy due to its motion.
Potential energy can be converted to kinetic energy and vice versa. For instance, when the ball is dropped, its potential energy is converted to kinetic energy as it gains speed due to gravity. The total mechanical energy remains constant in such energy transformations if we neglect frictional losses.
Difference Between Heat and Energy
Heat and energy are two related but distinct concepts in thermodynamics. The main difference is that heat refers to the transfer of thermal energy between systems, while energy is an intrinsic property of a system related to its capacity to do work.
More specifically, heat is energy in transit. It flows spontaneously from objects at higher temperatures to objects at lower temperatures. For example, when you put your hand on a hot stove, heat transfers from the stove (high temperature) to your hand (low temperature), causing your hand to get hotter.
In contrast, energy is a property possessed by objects and systems. It is the capacity to do work or cause change. Some common forms of energy are kinetic energy, potential energy, and internal energy. An object’s internal energy comes from the kinetic and potential energy of its molecules. This internal energy is the total energy contained within the object.
So in summary, heat is a process of thermal energy transfer between systems, while energy is a property intrinsic to a system related to its capacity for doing work. Heat involves energy flowing from one system to another, while energy is wholly contained within a system.
Measuring Heat vs Energy
While heat and energy are related concepts in thermodynamics, they are measured using different units.
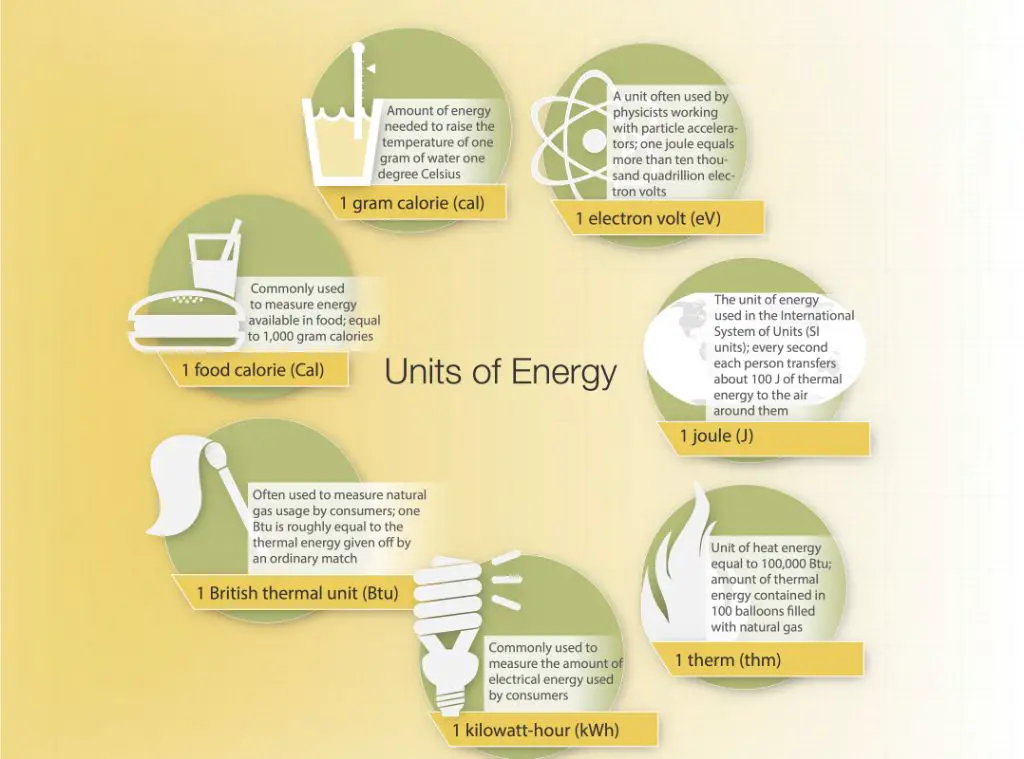
Heat is measured in units of calories or joules. A calorie is the amount of heat required to raise the temperature of 1 gram of water by 1°C. A joule is another metric unit of energy, defined as the work required to produce one watt of power for one second.
Energy is measured in joules, calories, kilowatt-hours, or other energy units. For example, the kinetic energy of an object can be calculated using its mass and velocity. The potential energy of an object depends on its mass, height, and the force of gravity. Electrical energy is commonly measured in kilowatt-hours.
While energy is conserved and can be transformed between different forms, heat is a transfer of thermal energy between objects due to temperature differences. Heat flows spontaneously from a hotter to a colder body. Measuring the heat transfer gives information about the thermodynamic process and interactions between the objects.
Calculations are done using the measured values for heat, work, and internal energy changes to determine quantities like efficiency, work output, and heat loss in thermodynamic systems and processes. The laws of thermodynamics govern the relationships between heat transfer and energy transformations.
Heat Transfer
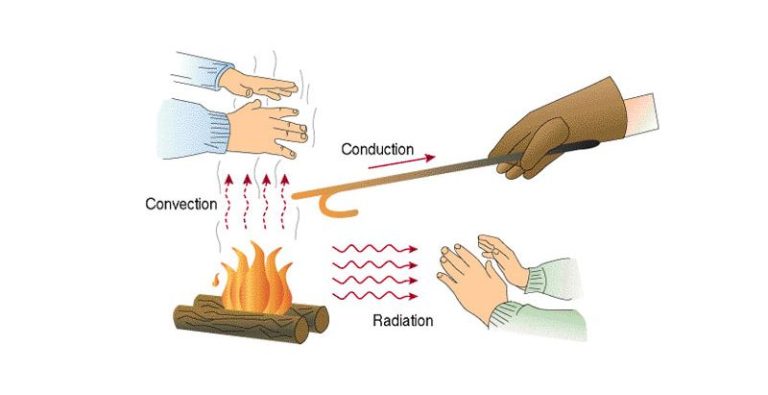
Heat transfer refers to the movement of heat or thermal energy from one object or system to another due to temperature differences. There are three main mechanisms of heat transfer: conduction, convection, and radiation.
Conduction is the transfer of heat between substances that are in direct contact with each other. It occurs when faster moving hot molecules collide with slower moving cold molecules, transferring kinetic energy. Metals are good conductors as their free electrons can transfer thermal energy rapidly.
Convection is the mode of heat transfer through fluids (liquids and gases). It occurs due to the circulation or bulk movement of molecules in fluids. As the fluid is heated, it expands, becomes less dense, and rises. Cooler denser fluid then moves to take its place, creating circulation. Convection can be natural (due to temperature differences) or forced (with fans or pumps).
Radiation is the transfer of heat via electromagnetic waves or photons. It does not require direct contact between objects. Radiative heat can travel through empty space. All objects emit thermal radiation depending on their temperature. Warmer objects emit more radiation than cooler objects. Radiation is the primary mode of heat transfer in a vacuum.
Energy Transformation
Energy can transform between different forms such as potential energy and kinetic energy. Potential energy is stored energy based on an object’s position or configuration. For example, a ball held at a height above the ground has gravitational potential energy due to its position. Kinetic energy is energy associated with motion. When the ball is dropped, the potential energy is converted into kinetic energy as the ball accelerates under gravity. The faster the ball falls, the greater its kinetic energy.
Other examples of energy transforming between potential and kinetic energy include:
- A compressed spring has potential energy. When released, the spring decompresses and kinetic energy is produced as it moves.
- Water held behind a dam has potential energy due to its elevated position. As water flows through the dam, the potential energy is converted into kinetic energy.
- Chemical bonds store potential energy. This can be released as kinetic energy during chemical reactions.
The law of conservation of energy states that the total energy in a closed system remains constant. Energy is never created or destroyed, just transformed from one form into another.
Laws of Thermodynamics
The laws of thermodynamics are fundamental principles that describe the behavior of heat and energy. There are four laws of thermodynamics that relate to the difference between heat and energy:
Zeroth Law of Thermodynamics
The zeroth law of thermodynamics states that if two systems are in thermal equilibrium with a third system, then they are also in thermal equilibrium with each other. This law helps define temperature and thermal equilibrium.
First Law of Thermodynamics
The first law of thermodynamics, also known as the law of conservation of energy, states that energy can neither be created nor destroyed – it can only be transformed from one form to another. This means that heat is a form of energy transfer, not a substance itself.
Second Law of Thermodynamics
The second law of thermodynamics states that the entropy of an isolated system always increases over time. Entropy is a measure of disorder, so this law describes how isolated systems tend to evolve toward thermal equilibrium and maximum disorder. This relates to the difference between heat and energy because heat transfer naturally flows from hot to cold.
Third Law of Thermodynamics
The third law of thermodynamics states that the entropy of a pure crystalline substance approaches zero as its temperature approaches absolute zero. This helps define the limits of converting heat into energy.
Together, these laws describe the fundamental relation between heat and energy in thermodynamic systems. The key difference is that heat is a transfer of thermal energy driven by temperature differences, while energy is the capacity to do work.
Real World Examples
Heat and energy are closely linked concepts that are applied in many real world systems like engines and heating systems.
In car engines, the exploding fuel provides energy to move the pistons. This fuel also generates a large amount of heat that the engine has to dissipate through the cooling and exhaust systems. The energy from the fuel gets converted into mechanical energy to turn the wheels, while the leftover heat is an example of wasted energy. Engineers aim to design more efficient engines that convert more of the fuel’s energy into useful work.
In home heating systems, the goal is to transfer heat energy from a source like natural gas, which provides the high temperature heat energy, into the home to raise the temperature. This requires an energy input to start and run the furnace, but the end goal is the transfer of heat. Energy transformations happen along the way, like from chemical energy in the gas to heat energy to the warmth in the home. But the focus is on getting the right amount of heat into the spaces.
In both examples, heat and energy are related but play different roles. Heat is the transfer of thermal energy between objects, while energy is the capacity to do work. Real world systems leverage both concepts in tandem.
Conclusion
In summary, while heat and energy are related concepts in thermodynamics, there are key differences between the two that are important to understand. Heat is a form of energy transfer between systems or objects due to temperature difference. It flows spontaneously from higher temperature to lower temperature systems. Energy is the capacity to do work and can exist in many forms like kinetic, potential, chemical, thermal, electrical, etc. Energy is conserved according to the First Law of Thermodynamics, while heat is not necessarily conserved.
Distinguishing between heat and energy is crucial in analyzing thermodynamic processes. The flow of heat and the transformation between different energy types drives thermal systems and heat engines. Properly differentiating heat transfer from energy transformation is key to applying the fundamental laws of thermodynamics and performing quantitative calculations. Having a solid grasp of the differences allows thermodynamic analysis of complex systems and processes, from power generation to refrigeration.