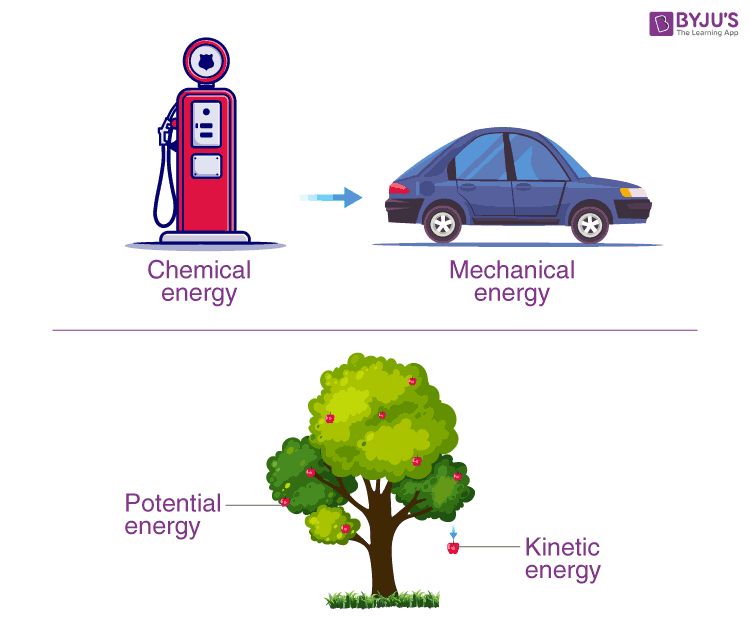
What Is Potential Energy At The Molecular Level?
Potential energy is the stored energy that an object has due to its position or chemical configuration. At the molecular level, potential energy arises from the particular arrangement of atoms, bonds, angles, and conformations within a molecule. This energy is not observed as motion, but instead reflects the possibility of future motion if the molecular structure changes.
The concept of molecular potential energy is foundational in chemistry and biochemistry. By understanding potentials and how molecular structures store energy, we gain insight into chemical reactions, protein folding, drug binding, and more. This introduction will explore the basics of molecular potential energy and its applications across chemistry and biology.
Potential Energy in Chemistry
In chemistry, potential energy arises from the arrangement and interactions between atoms and molecules. The most fundamental source of chemical potential energy is the bonding energy between atoms within molecules and between separate molecules.
For example, energy is required to break the covalent bonds that hold atoms together in a molecule. This energy to break the bond is equal to the potential energy stored in the bond itself. Stronger bonds like triple bonds have higher potential energy than weaker single bonds. The type of atoms involved also impacts the bond energy. Bonds between atoms with high electronegativity have higher potential energy.
Potential energy is also stored in the spatial arrangement of atoms and shared electron pairs in a molecule. Atoms can interact through charge-charge, dipole-dipole, and induced dipole interactions based on their molecular geometry. For example, nonpolar molecules have lower potential energy than polar molecules which have permanent dipole moments. The orientation of polar molecules also impacts their potential energy.
Intermolecular attractive forces like hydrogen bonding and van der Waals forces also give rise to potential energy between separate molecules. Overcoming these attractive forces requires energy, which is then stored as potential energy in the system.
In summary, the potential energy in chemistry stems from electronic, electrostatic, and steric effects between atoms and molecules. This energy can be estimated through quantum mechanical models and measurements.
Potential Energy from Molecular Conformation
Molecules can store potential energy based on their shape and structure, known as their conformation. The bonds between atoms in a molecule act like springs, and altering the angles and distances between the atoms requires energy. For example, rotating a bond or distorting a bond angle away from the preferred geometry results in an increase in potential energy as the molecular bonds are strained.
The amount of energy stored depends on how far the conformation is distorted from the optimal geometry, which is often the lowest energy state. Bringing a molecule into a higher energy conformation requires the input of energy, which is then stored as potential energy. For example, the linear structure of carbon dioxide is a lower energy state than the bent structure.
Molecules with freely rotating single bonds, such as ethane (CH3-CH3) can adopt different conformations based on rotation around the central C-C bond. These rotational isomers represent distinct potential energy states for the same molecule. Proteins and enzymes also rely heavily on conformational changes to regulate their function and activity through transitions between higher and lower potential energy states.
Potential Energy in Biomolecules
Potential energy plays an important role in biomolecules like proteins and DNA. Biomolecules are composed of hundreds to thousands of atoms bonded together, and the atoms have a set configuration based on their chemical bonds. However, biomolecules are flexible structures and their atoms are often able to move around and rotate to some degree. This allows the overall conformation and shape of proteins, DNA, and other biomolecules to change.
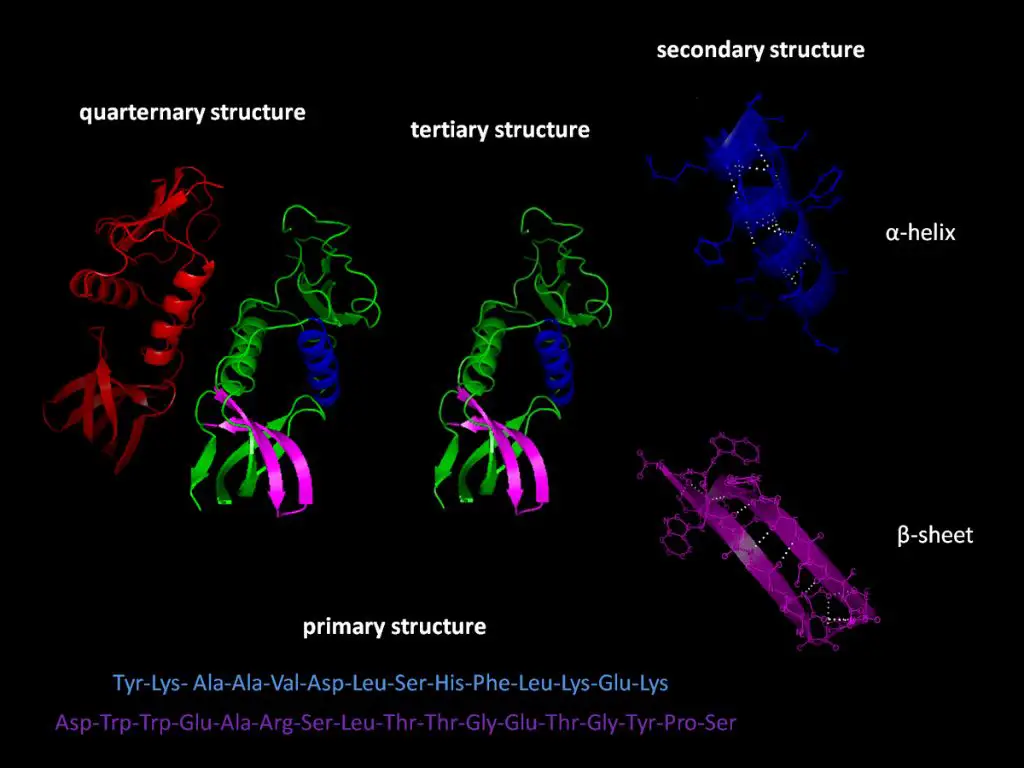
The different possible conformations of a biomolecule represent different potential energy states. For example, a protein can fold and unfold into many possible shapes, each with a different amount of potential energy. The folded state generally has the lowest potential energy, while an unfolded state has higher potential energy due to strain on the bonds. Transitions between conformations require energy input to overcome the potential energy barriers, which allows proteins to respond to cellular signals and change shape as needed to carry out functions.
In DNA, the two DNA strands can unwind and separate, increasing their potential energy as the hydrogen bonds between base pairs are broken. This strand separation is required during DNA replication and transcription. The amount of potential energy stored in a DNA double helix is enormous, equivalent to the energy in an entire liter of gasoline. This illustrates the huge quantities of potential energy stored within biomolecular structures.
Overall, changes in molecular conformation allow biomolecules to cycle between high and low potential energy states. This storage and release of potential energy powers many essential biological processes.
Measuring Molecular Potential Energy
There are several methods used to measure the potential energy of molecules and molecular systems. Some key techniques include:
Spectroscopy: Spectroscopic techniques like infrared, Raman, and ultraviolet-visible spectroscopy can provide information about the energy levels of molecules. By measuring the frequencies of absorbed or emitted photons, the energy differences between ground and excited states can be determined. These correspond to the potential energy surface of the molecule.
Scanning probe microscopy: Methods like atomic force microscopy and scanning tunneling microscopy can map the potential energy surface of a molecule by measuring forces and currents as the probe tip interacts with the surface. This provides nanoscale resolution of molecular potential energies.
Calorimetry: By carefully measuring the heat absorbed or released during chemical reactions or phase changes, the associated changes in potential energy can be quantified. For example, a molecule’s lattice energy (from crystal formation) or vaporization energy can be measured.
Computational chemistry: The potential energy surface of a molecule can be calculated theoretically using methods like density functional theory and high-level ab initio techniques. This provides quantitative predictions that can complement experimental data.
Overall, spectroscopy, microscopy, calorimetry, and computations provide complementary information about molecular potential energies, giving a detailed picture of this important molecular property.
Modeling Potential Energy
Computational modeling methods are frequently used to understand and analyze molecular potential energy. These methods allow researchers to calculate the potential energy surfaces of molecular systems using quantum mechanical calculations. Some commonly used computational methods for modeling molecular potential energy include:
- Molecular mechanics – Uses classical physics to model bonded and non-bonded interactions.
- Semi-empirical methods – Approximates some quantum mechanical integrals to reduce computational cost.
- Ab initio methods – Solves the electronic Schrödinger equation from first principles.
- Density functional theory – Models the electron density rather than wavefunctions.
These computational modeling methods allow researchers to map out the potential energy surfaces of molecules as they undergo conformational changes or chemical reactions. The calculated energies provide insights into molecular structure, stability, reactivity, and other properties related to the potential energy. Some specific applications include calculating reaction pathways, modeling molecular dynamics, protein folding simulations, and computational drug design.
Applications and Impacts of Molecular Potential Energy
Molecular potential energy has many practical uses and implications across a diverse set of fields including chemistry, biology, physics, engineering, and more. Understanding potential energy at the molecular level enables advances in areas such as:
Drug design – Knowledge of potential energy surfaces for drug molecules allows medicinal chemists to predict and control the 3D structure of drugs. This enables designing drugs that bind with high specificity to target receptors.
Materials science – Tailoring molecular potential energy profiles allows control over properties like mechanical strength, conductivity, and reactivity in materials like polymers and nanomaterials.
Catalysis – Shaping reaction energy landscapes through catalysts with specific molecular conformations allows lowering of activation barriers and speeding up rates for chemical manufacturing.
Molecular machines – Molecular potential energies can be exploited to create nanoscale devices that harness molecular motion and forces to do work like delivery drugs or sense signals.
Renewable energy – Potential energy surfaces guide optimization of solar energy capture and conversion through photosynthetic proteins and photocatalysts.
Climate change – Understanding greenhouse gas potential energy diagrams informs strategies to catalytically convert them to less harmful forms.
In summary, knowledge of molecular potential energy provides scientists and engineers with a powerful toolkit for innovating solutions in many technology domains, with profound impacts on improving human health, sustainability, and quality of life.
Potential Energy Curves
Potential energy diagrams, also known as potential energy surfaces, are graphical representations that illustrate the potential energy of a molecule or collection of atoms relative to the molecular geometry or atomic positions. They provide critical insights into chemical reactions, molecular dynamics, and thermodynamic properties.
For a simple diatomic molecule, a potential energy curve plots the potential energy as a function of the internuclear distance. The x-axis shows the distance between the two atoms, while the y-axis shows the associated potential energy. At large internuclear distances, the potential energy is low, representing the dissociated atoms. As the atoms approach each other, the potential energy drops to a minimum, representing the equilibrium bond length. At very short internuclear distances, the potential energy increases rapidly as a result of repulsive forces between the electron clouds.
For larger molecules, potential energy surfaces plot the potential energy as a function of multiple structural coordinates, providing a multidimensional surface. The topology of the surface provides key information about molecular conformations, transition states, and reaction mechanisms. Potential energy surfaces allow researchers to model processes like protein folding, catalytic activity, and molecular collisions.
Advanced quantum mechanical calculations are required to accurately model potential energy surfaces. The surfaces help explain experimental results and make predictions about molecular properties and chemical reactions. By mapping out the peaks and valleys of a potential energy landscape, scientists gain critical insights into the forces and dynamics that drive molecular behavior.
Kinetic Energy Relationship
Potential and kinetic energy have an inverse relationship at the molecular level. As potential energy decreases, kinetic energy increases and vice versa. This interconversion between potential and kinetic energy forms the basis for chemical reactions and biological processes.
Potential energy is stored energy. When some external factor activates a molecule, allowing it to relax to a lower energy conformation, the released potential energy is converted into kinetic energy. Essentially, the molecule speeds up due to the added energy. In chemistry, this can enable atoms to collide and form new bonds during a reaction. In biology, it allows motor proteins to move by rapidly hydrolyzing molecules like ATP.
The process can also work in reverse. As molecules collide and react, their random kinetic energy gets focused into potential energy stored in the new chemical bonds. The same goes for motor proteins exerting mechanical force to convert chemical energy from ATP into the potential energy of a tensed muscle fiber.
This continual interplay between potential and kinetic energy provides the power to drive biochemical systems at the molecular level. By modeling it mathematically, scientists gain insights into reaction mechanisms, protein motion, and other essential processes.
Conclusions
In summary, potential energy at the molecular level refers to the stored energy within a molecule’s structure and bonds. This energy can be released as molecules change shape or break bonds. Understanding potential energy is key for interpreting chemical reactions and biological processes.
Going forward, researchers can continue refining computational methods to model potential energy more accurately. New techniques may enable more precise predictions about molecular behaviors. Quantifying potential energy also aids emerging fields like nanotechnology and drug design.
Overall, grasping molecular potential energy provides insights into chemistry, biology, physics and materials science. It underpins explanations of complex phenomena at the atomic scale. Scientists can apply this knowledge to develop novel applications and technologies. So comprehending the basics of potential energy at the molecular level remains an essential endeavor with profound scientific and practical impacts.