Does An Exergonic Reaction Release Energy?
Definition of an Exergonic Reaction
An exergonic reaction is a chemical reaction where the reactants have more free energy than the products. Free energy is a thermodynamic property that measures the amount of energy available to do work in a system. In an exergonic reaction, as the reaction proceeds towards products, energy is released, often in the form of heat. This release of energy makes the products have lower free energy than the reactants.
The difference in free energy between the reactants and products is called the change in Gibbs free energy (ΔG). For an exergonic reaction, ΔG is a negative value, meaning the products have less free energy than the reactants. This negative ΔG indicates the reaction releases free energy and is thermodynamically favorable.
Exergonic reactions involve the conversion of some form of energy that is “locked up” in the reactants into heat that is released into the surroundings. This drives the reaction forward spontaneously. The released energy can be captured to perform useful work, such as in ATP synthesis during cellular metabolism.
In summary, an exergonic reaction proceeds with a net release of free energy. The reactants contain more chemical potential energy than the products, resulting in a negative ΔG and a spontaneous, thermodynamically favorable reaction.
Energy Profiles of Exergonic Reactions
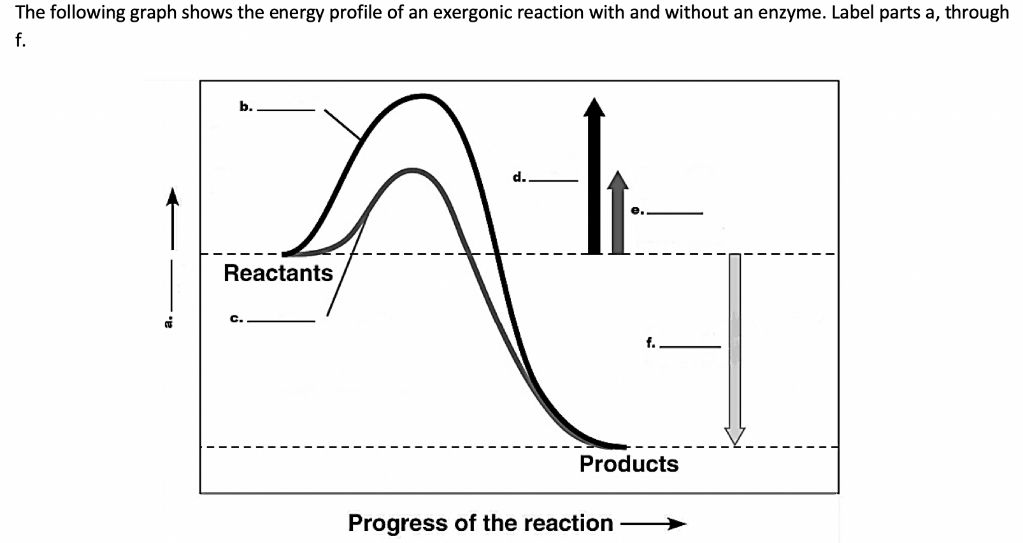
Exergonic reactions proceed spontaneously, releasing free energy. The energy profile of an exergonic reaction can help illustrate this energy release. The energy profile shows how the energy of the reactants and products changes during the reaction.
On the energy profile, the x-axis represents the progress of the reaction from reactants to products. The y-axis represents the free energy. The reactants start at a higher free energy than the products. There is an activation energy barrier that must be overcome for the reaction to proceed.
Once enough energy is added to overcome this activation barrier, the reaction proceeds downhill towards forming the products. As the bonds break in the reactants and reform in the products, energy is released. This makes the free energy of the products lower than the reactants, resulting in an overall negative change in free energy.
The energy profile visually shows how exergonic reactions spontaneously proceed once initiated, steadily releasing energy as they form products. The activation energy represents the energy input needed to start the downhill process. But after that point, no more energy input is required for the reaction to continue.
Examples of Exergonic Reactions
Some common examples of exergonic reactions include:
-
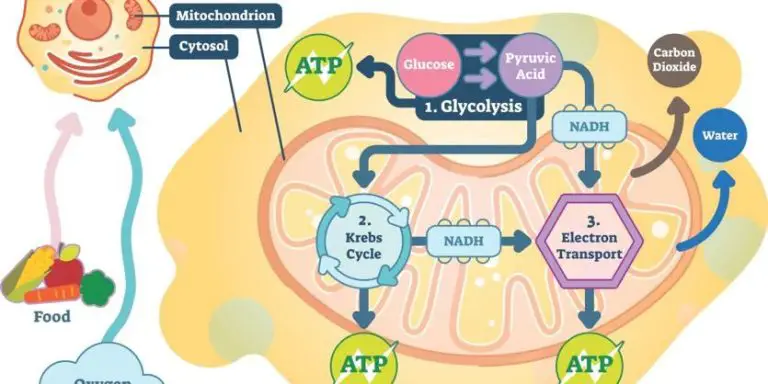
Cellular respiration – The process by which cells convert nutrients like glucose into energy in the form of ATP. Cellular respiration is an exergonic process involving a series of chemical reactions.
-
Digestion – The breakdown of large food molecules like proteins, fats and carbohydrates into smaller molecules that can be absorbed and utilized by the body. Digestion involves many exergonic reactions catalyzed by enzymes that split bonds in food molecules.
-
Combustion – The burning of fuels like wood, coal, oil etc in presence of oxygen is an extremely exergonic process. A lot of energy is released which can be captured in the form of heat.
-
Battery reactions – The conversion of chemical energy into electrical energy in batteries involves exergonic redox reactions. As electrons flow from the anode to the cathode through the external circuit, energy is released.
-
Explosions – Explosives contain unstable chemical compounds that can undergo rapid decomposition reactions that are highly exergonic, releasing tremendous amounts of energy.
These are just a few examples of common exergonic processes. Many chemical reactions taking place around us, within our bodies and in the environment are exergonic in nature.
Measuring Free Energy Changes
The free energy change (ΔG) of a chemical reaction indicates whether the reaction is exergonic or endergonic. Exergonic reactions have a negative ΔG, meaning they release free energy. Endergonic reactions have a positive ΔG, meaning they absorb free energy.
The ΔG of a reaction can be measured experimentally to determine if the reaction is exergonic or endergonic. This is done by measuring the standard free energy change (ΔG°) under standard conditions of temperature, pressure and concentration.
ΔG° provides a quantitative measure of whether a reaction will proceed spontaneously under standard conditions. If ΔG° is negative, the reaction is exergonic and will proceed spontaneously. If ΔG° is positive, the reaction is endergonic and will not occur without an input of energy.
To experimentally determine ΔG°, the equilibrium constant (Keq) of the reaction is first measured. Keq indicates the ratio of products to reactants at equilibrium. Using Keq, ΔG° can then be calculated using the equation:
ΔG° = -RT ln Keq
Where R is the gas constant and T is the absolute temperature in Kelvin. So by measuring Keq for a reaction and plugging it into this equation, chemists can quantitatively determine if the reaction is exergonic or endergonic under standard conditions. This allows them to predict whether the reaction will release energy or require an energy input to proceed.
Exergonic Reactions Release Energy
Yes, exergonic reactions do release energy as they proceed spontaneously. Exergonic reactions have a negative change in Gibbs free energy (ΔG), indicating that the products are at a lower energy state than the reactants. As the reaction proceeds towards equilibrium, moving from a higher energy state to a lower one, energy is released in the form of heat, light, or sound.
The release of energy is what drives exergonic reactions to completion. It is thermodynamically favorable for the system to move to a state of lower energy, so these reactions can occur spontaneously without any external energy input. The amount of energy released equals the magnitude of the negative ΔG value for that reaction.
Some common examples of exergonic reactions that clearly demonstrate an energy release include combustion reactions, explosive reactions, and many enzyme-catalyzed reactions in cells. The energy released can be harnessed to perform useful work. Overall, the defining feature of an exergonic reaction is that it results in products that are at a lower energy state than the starting reactants.
Uses of Exergonic Reactions
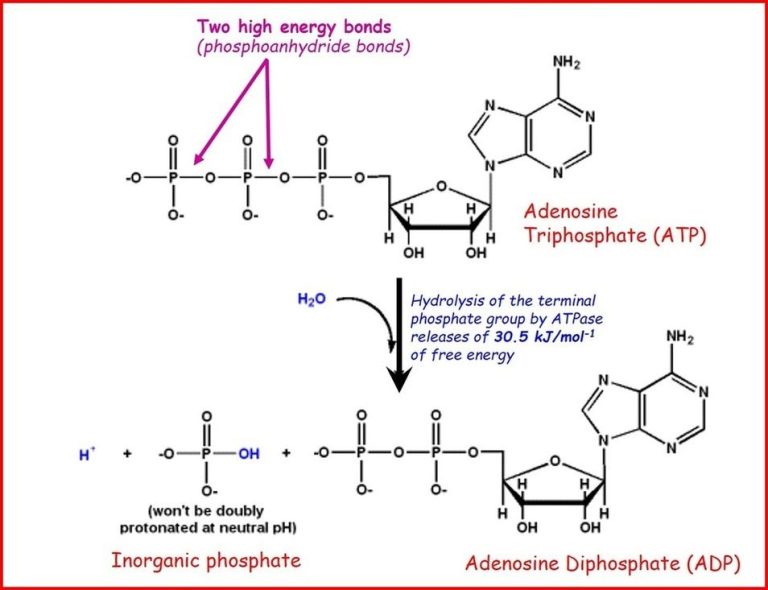
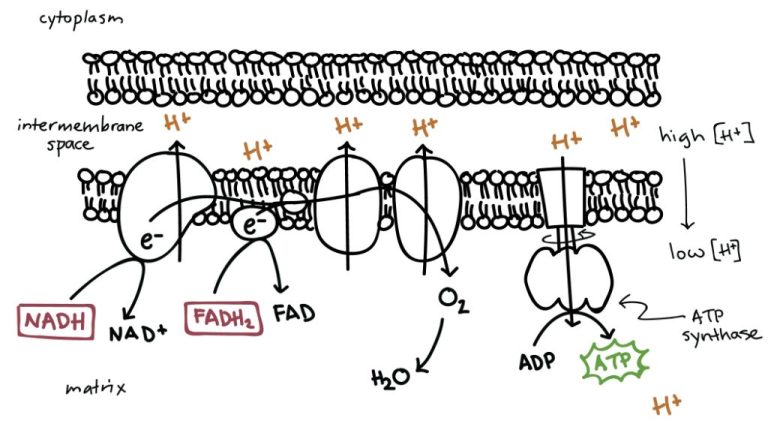
Cells harness the energy released from exergonic reactions to perform work. One of the most important exergonic reactions in cells is the hydrolysis of adenosine triphosphate (ATP) to adenosine diphosphate (ADP) and inorganic phosphate (Pi). This reaction releases about 30.5 kJ/mol of energy under standard conditions. Cells use this released energy to power critical functions like biosynthesis, transport, and mechanical work.
For example, muscle contraction relies on ATP hydrolysis. The myosin heads in muscle fibers bind to actin filaments and change conformation, generating force and causing the filaments to slide over one another, shortening the muscle. This process requires energy input from ATP hydrolysis. The energy released allows myosin to change shape and interact with actin, generating the force for contraction.
Another example is active transport across membranes. Moving substances against their concentration gradients requires energy, which cells derive from ATP hydrolysis. The Na+/K+ ATPase, for instance, uses the energy from ATP hydrolysis to pump sodium ions out of the cell and potassium ions in. This generates concentration gradients that are critical for functions like nerve conduction and muscle contraction.
In summary, exergonic reactions like ATP hydrolysis provide the energy that allows cells to perform mechanical work, biosynthesis, active transport, and other processes requiring an input of energy. Cells have evolved to couple exergonic and endergonic reactions through energy carriers like ATP to meet their energy needs.
Contrast with Endergonic Reactions
In contrast to exergonic reactions that release energy, endergonic reactions require energy input to proceed. Endergonic reactions absorb free energy from their surroundings, usually in the form of heat, electrical, or light energy. They have a positive change in free energy (ΔG > 0).
A simple example is photosynthesis, where plants absorb light energy from the sun to convert carbon dioxide and water into glucose and oxygen. This reaction has a positive ΔG and will not occur spontaneously without the energy input from sunlight. Other endergonic reactions in biology include active transport of molecules across cell membranes and muscle contraction.
The energy absorbed by endergonic reactions enables them to synthesize larger, more complex molecules from simpler starting materials. This synthesized energy-rich biomass can then fuel exergonic reactions. Endergonic and exergonic reactions are coupled together in cells to drive biochemical pathways.
Role in Metabolism
Exergonic and endergonic reactions are coupled together in metabolism to allow energetically unfavorable reactions to proceed. Metabolism involves many chemical reactions that are critical for sustaining life, including the breakdown of nutrients to generate energy and the biosynthesis of complex molecules needed by the cell.
Many of the chemical reactions involved in metabolism are endergonic, meaning they require an input of energy to proceed. Examples include the synthesis of large molecules like proteins, lipids, and nucleic acids from smaller precursors. On their own, these endergonic reactions are energetically unfavorable.
To drive these critical but energetically unfavorable reactions, cells couple them to exergonic reactions that release free energy. The exergonic reactions provide the input of energy needed to proceed with the coupled endergonic reactions. A common example is the breakdown of glucose in glycolysis, which is an exergonic pathway that releases energy. This energy is then coupled to drive endergonic reactions like the synthesis of ATP.
By coupling exergonic and endergonic reactions, cells can carry out the metabolically important but energetically unfavorable reactions needed to sustain life. The sequences of coupled reactions form metabolic pathways that allow cells to capture, transform, and harness energy from nutrients.
Equilibrium Constant
The equilibrium constant (K) is directly related to the change in Gibbs free energy for a reaction. For an exergonic reaction where the change in Gibbs free energy (ΔG) is negative, the equilibrium constant will be greater than 1.
This makes sense based on Le Chatelier’s principle. Since an exergonic reaction releases energy, it proceeds spontaneously in the forward direction. At equilibrium, the reaction favors the formation of products over reactants. Therefore, the equilibrium constant for an exergonic reaction will be greater than 1.
The precise mathematical relationship is: ΔG° = -RT ln K. R is the gas constant, T is temperature in Kelvin, and K is the equilibrium constant. As ΔG° becomes more negative for a highly exergonic reaction, ln K becomes larger and K itself becomes much greater than 1. So a large, positive K value indicates an exergonic reaction.
In summary, the equilibrium constant provides quantitative information about the spontaneity of a reaction. An exergonic reaction with a negative ΔG will have an equilibrium constant larger than 1.
Summary
In summary, exergonic reactions release free energy as they proceed spontaneously, providing energy to do useful work. An exergonic reaction has a net negative free energy change, meaning the products have lower free energy than the reactants. This free energy is released when the reaction occurs, often in the form of heat. Exergonic reactions are critical in biology, as organisms harness their energy to perform essential functions and processes. Examples include cellular respiration, where glucose is oxidized to release energy, and photosynthesis, where light energy is used to build carbohydrates. The spontaneous and energy-releasing nature of exergonic reactions makes them thermodynamically favorable and essential drivers of chemistry both within and outside of living systems.