Is Energy The Energy We Can See?
Energy is a fundamental concept in physics that describes the capacity to do work and cause change. Though energy manifests in many visible forms, like the kinetic energy of a moving car or the light radiating from the sun, energy itself is an invisible property of matter and radiation. The goal of this article is to explore the different fundamental forms that energy takes, including those beyond what we can directly see with our eyes.
Kinetic Energy
Kinetic energy is the energy of motion. When an object is in motion, it has kinetic energy. The faster an object moves, the more kinetic energy it possesses. Some examples of kinetic energy include:
- A baseball being thrown or hit
- A car driving down the road
- Wind blowing
- A person walking or running
The amount of kinetic energy depends on the mass and speed of the moving object. The formula for kinetic energy is:
Kinetic Energy = 1/2 x mass x velocity^2
This shows that an object’s kinetic energy increases exponentially as its speed increases. Kinetic energy is directly proportional to mass as well. Therefore, a more massive object moving at the same speed as a less massive object will have more kinetic energy.
When objects collide or interact, kinetic energy can be transferred between them or converted into other forms of energy like potential energy. Understanding kinetic energy is important for analyzing mechanical systems and phenomena from throwing a ball to designing car safety features.
Potential Energy
Potential energy is the stored energy an object has due to its position or arrangement. There are several types of potential energy:
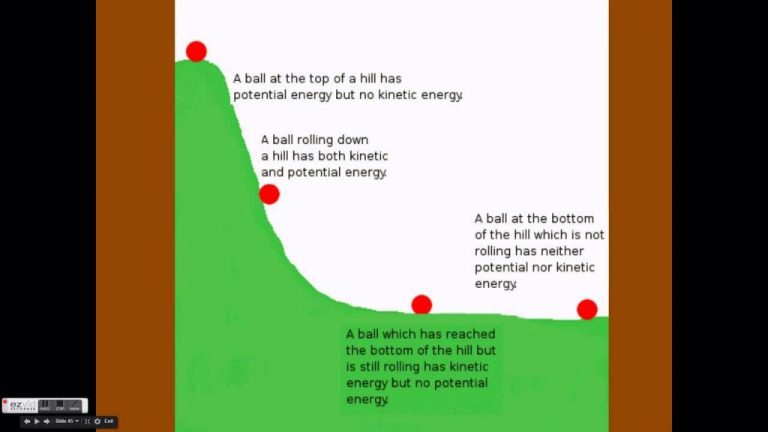
- Gravitational Potential Energy – This is energy stored due to an object’s height. For example, a book on a high shelf has more gravitational potential energy than a book on a low shelf. As the book falls, its potential energy is converted to kinetic energy.
- Elastic Potential Energy – When a spring or rubber band is stretched or compressed, it stores elastic potential energy. This energy is converted to kinetic energy when released.
- Chemical Potential Energy – Energy stored in the bonds between atoms and molecules is chemical potential energy. This energy can be released during chemical reactions to produce heat.
- Nuclear Potential Energy – Energy stored in an atom’s nucleus is nuclear potential energy. It can be released in nuclear fission or fusion reactions.
Potential energy is not actually “energy” in the same sense as kinetic energy. It is better thought of as a property of a system that has the potential to gain or release energy due to its configuration.
Chemical Energy
Chemical energy is the potential energy stored in the bonds between atoms and molecules. It is the energy that holds these particles together. There are two types of chemical energy:
-
Potential energy – this is energy stored in the chemicals. Examples include energy stored in molecules like sugars, fats, and gasoline.
-
Kinetic energy – this is energy released when chemical bonds are broken. Examples include burning wood, digesting food, and combustion of fuel in an engine.
Chemical energy is converted into thermal energy when bonds are broken. The stored potential energy is released as heat. Foods and fuels like wood, oil, and natural gas all contain chemical energy in their molecular bonds. When we eat food, digesting it breaks chemical bonds and releases energy our bodies use. Burning wood breaks bonds in the molecules and releases thermal energy we feel as heat. Gasoline, batteries, and dynamite sticks all contain chemical energy in their atomic bonds too.
Chemical energy is an extremely useful form of stored energy. It allows us to capture energy from the sun and use it later for heating and electricity. Plants use photosynthesis to convert solar energy into the chemical energy stored in wood and biofuels. We release that stored energy by burning those molecules. Batteries also convert chemical energy into electricity through redox reactions. Chemical energy makes an excellent transportable and high-density store of energy.
Electrical Energy
Electrical energy is the energy derived from the movement of charged particles, such as electrons. It is a form of energy that can be generated through various natural and man-made processes and has become an essential part of modern society.
Some examples of electrical energy in action include:
-
Lightning – The massive buildup and discharge of electrical energy during a thunderstorm.
-
Electric eels – These fish can generate up to 600 volts of electricity to stun prey or defend themselves.
-
Batteries – Portable devices that store chemical energy and convert it into electrical energy to power electronics.
-
Power plants – Facilities like dams, nuclear reactors, or solar farms that produce electricity on a large scale.
-
Electrical outlets – Provide access to the electrical grid so we can plug in appliances and devices.
-
Defibrillators – Used to transmit electric shocks to restart the heart during cardiac arrest.
As a highly useful and versatile energy form, electricity powers countless aspects of our lives at home, work, and everywhere in between. Our modern society truly runs on electrical energy.
Nuclear Energy
Nuclear energy is the energy released from the nucleus of an atom through nuclear fission or fusion. In nuclear fission, atoms like uranium or plutonium are split into smaller atoms, releasing a large amount of energy in the process. Nuclear power plants use nuclear fission to heat water into steam, which then spins turbines to generate electricity.
Nuclear fusion works by fusing together light atoms like hydrogen into heavier ones like helium. This process gives off even more energy than fission. The sun produces energy through fusion. While fusion holds great promise as an energy source, the technology to harness it is still in development.
Nuclear energy is extremely efficient, producing vastly more energy for its weight than coal or oil. However, it also creates radioactive waste that must be carefully stored. Nuclear energy is controversial, with proponents arguing it offers a reliable form of low-carbon energy, while opponents cite risks like accidents and proliferation of nuclear materials.
Thermal Energy
Thermal energy refers to the total internal kinetic energy and potential energy of atoms and molecules in an object. This energy results from the random motions of molecules and atoms in matter, and is sometimes referred to as heat energy. Thermal energy can be transferred between objects through processes like conduction, convection, and radiation.
For example, when you boil a pot of water on the stove, the burner provides thermal energy that is transferred to the pot and water, increasing the internal kinetic energy of the water molecules until they reach boiling point. The thermal energy causes the water molecules to move faster and spread apart, transitioning from liquid water to water vapor. Thermal energy can also flow from hotter objects to colder objects – for instance, a hot cup of coffee left on a table eventually transfers its thermal energy to the surrounding air, causing the coffee to cool down over time.
Other examples of thermal energy in everyday life include the warmth provided by the sun, friction heat generated when rubbing your hands together, and geothermal energy from the Earth’s hot interior that can be used to heat buildings or generate electricity.
Radiant Energy
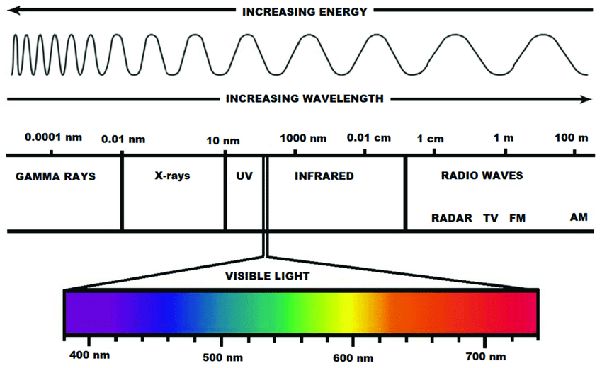
Radiant energy is energy that is transferred through electromagnetic waves or photons. It does not rely on particles or matter to transfer the energy. Radiant energy includes visible light, ultraviolet light, infrared energy, radio waves, X-rays, and gamma rays. These different forms of radiant energy occupy different positions on the electromagnetic spectrum, which arranges electromagnetic radiation by frequency and wavelength.
Some key examples of radiant energy in everyday life include:
- Sunlight – The light and warmth we feel from the Sun is radiant energy in the form of visible light and infrared radiation.
- Microwaves – Microwave ovens use radiant energy in the microwave frequencies to heat up food.
- Radio waves – Radios and cell phones rely on radio wave radiation to transmit data and communicate.
- X-rays – Doctors use X-ray radiation to take images of bones and other internal body structures.
Radiant energy can travel long distances and does not require any medium to transfer it. It exhibits wave-like behavior but also demonstrates properties of particles (photons). Radiant energy is different from other forms of energy like thermal, electrical, chemical, and nuclear energy that rely on the motion or interaction of particles and matter to transfer the energy.
Discussion
Energy exists all around us in many different forms, some visible and some invisible. The most obvious forms of energy that we can see directly are kinetic energy, the energy of motion, and potential energy, the stored energy of an object due to its position or state. For example, we can see a ball flying through the air with kinetic energy, or a rock sitting at the edge of a cliff with potential energy ready to fall.
However, there are many important forms of energy that are not visible to our eyes. Chemical energy is energy stored in the bonds between atoms and molecules. Batteries, fuels, and food all contain chemical energy that is released in chemical reactions. Electrical energy is the movement of electrons which we cannot see directly but is responsible for powering all electronics around us. Nuclear energy comes from processes within atomic nuclei and drives nuclear power plants. Thermal energy arises from the motion of atoms and molecules and manifests as heat. These types of energy are invisible but essential sources of power for human civilization.
Radiant energy, including visible light, ultraviolet rays, infrared radiation, microwaves, and radio waves, can transport energy through electromagnetic waves. Though we cannot see many parts of the electromagnetic spectrum, they carry energy through space at the speed of light. The visible light from the Sun is a very familiar example of radiant energy.
In summary, energy is all around us, embodied in visible motion like a moving object, invisible fields like magnetism, stored in matter at atomic and molecular scales, and transmitted by waves across space. Appreciating the diverse forms energy takes, even those beyond our senses, allows us to better understand and harness energy to improve our lives.
Conclusion
This article has covered the different forms that energy can take. We started by defining energy as the ability to do work or produce change. While kinetic and potential energy are the forms we can readily see and feel in our everyday lives, there are many other important types of energy that may be less visible.
Chemical, electrical, nuclear, thermal and radiant energy power our world even though they operate at the atomic and molecular level. From the chemical energy in the food we eat, to the thermal energy that heats our homes, to the nuclear reactions that fuel stars – energy is behind everything. What we can physically see and touch is just the surface.
The key takeaway is that energy is more than what meets the eye. Though kinetic and potential energy are the most tangible, many other forms exist that we rely on constantly. Energy is an invisible force that powers our universe in countless seen and unseen ways.