Is Heat Just Energy?
What is heat?
Heat is the transfer of thermal energy between substances due to a temperature difference. It refers specifically to the amount of energy that is transferred, rather than the temperature of the objects themselves. Heat is measured in joules, which are units of energy.
Heat should not be confused with temperature, although they are related concepts. Temperature measures how hot or cold an object is, while heat is the flow of thermal energy from a hotter object to a colder object until they reach thermal equilibrium. For example, a burner on a stove transfers heat to a pot of water, increasing the thermal energy of the water and thus raising its temperature.
In summary, heat is the transfer of thermal energy due to a temperature difference, measured in joules. It is different from temperature, which measures the hotness or coldness of an object. Understanding the difference between heat and temperature is key to understanding thermodynamic principles.
Relationship between heat and energy
Heat is a form of energy that manifests due to the motion of molecules and atoms. When two objects with different temperatures come into contact, heat transfer occurs as energy is transferred from the higher-temperature object to the lower-temperature object. This energy transfer continues until thermal equilibrium is reached and both objects are at the same temperature. Heat always flows spontaneously from hotter to colder matter.
On an atomic level, heat arises from the kinetic energy of atoms and molecules in matter. Increased molecular motion corresponds to an increase in temperature. So heat is not contained within an object, but rather reflects the total kinetic energy of all the atoms that make up an object. This kinetic energy can be transferred between objects through the processes of conduction, convection or radiation.
So in summary, heat is a manifestation of the energy stored within matter due to molecular motion. Heat transfer is the process by which this internal energy is spontaneously transferred from one object to another due to their temperature difference. This highlights the intrinsic relationship between heat and energy.
How heat transfers
There are three main methods of heat transfer:
Conduction
Conduction is the transfer of heat between substances that are in direct contact with each other. It occurs when faster moving atoms pass on thermal energy to neighboring slower atoms through oscillations and free electron collisions. Metals are good conductors because they contain many free electrons.
Convection
Convection is the transfer of heat by the movement of heated fluid or gas. As the fluid is heated, it expands, becomes less dense, and rises. Cooler denser fluid then moves to take its place, creating flow currents that transfer heat. Convection occurs in liquids like water and gases like air.
Radiation
Radiation is the transfer of heat not requiring a medium to travel through. It occurs when energetic particles or waves like infrared, visible light, or microwaves are emitted from hot objects and absorbed by cooler ones. No direct contact is needed. The sun warming the Earth is an example of heat transfer by radiation.
Effects of heat transfer
Heat transfer leads to several observable effects, like heating and cooling objects and facilitating changes in state between solid, liquid, and gas phases.
When heat transfers into an object, the added thermal energy makes the molecules vibrate and move faster, increasing their kinetic energy. This manifests as a rise in temperature. Heat transfers out of objects when they are in contact with lower temperature surroundings, decreasing molecular motion and kinetic energy. Materials expand when heated up due to increased molecular vibrations, and contract when cooled down as kinetic energy decreases.
Heating and cooling via heat transfer facilitates phase changes between solid, liquid, and gas states. Adding heat to solids increases molecular vibrations and kinetic energy until the molecules can break free of their fixed positions, melting the solid into a liquid state. Further heating increases intermolecular vibrations and motion until molecules can freely move apart into a gas phase through evaporation and boiling.
Reverse changes happen when heat transfers out of matter. As kinetic energy decreases, liquid molecules slow down and bind together into crystalline solid structures through freezing and condensation. Gases condense into liquid form by losing enough heat for molecules to stick together. The energy required to change states between solid, liquid, and gas is called latent heat.
Measuring heat
Heat is measured in a variety of units depending on the system being used. Some common units used to measure heat are:
- Joules – The joule is the SI unit of energy and work. It is also the standard unit of heat used in the sciences.
- Calories – The calorie is a non-SI unit of heat still widely used, especially in nutrition and chemistry. One calorie is the amount of heat required to raise the temperature of 1 gram of water by 1°C.
- British thermal units (BTUs) – The BTU is a traditional unit of heat still used today, especially in the United States. One BTU is the amount of heat required to raise the temperature of 1 pound of water by 1°F.
There are several devices used to precisely measure heat and temperature:
- Thermometers – Used to measure temperature. Different types like mercury, alcohol, and digital thermometers work in different ways.
- Calorimeters – Instruments designed to measure the heat transferred in a specific process. They help determine heats of reaction, phase changes, and more.
Having standardized ways to measure heat allows scientists to quantify energy transfers and make calculations using the proper units.
Heat flow and temperature
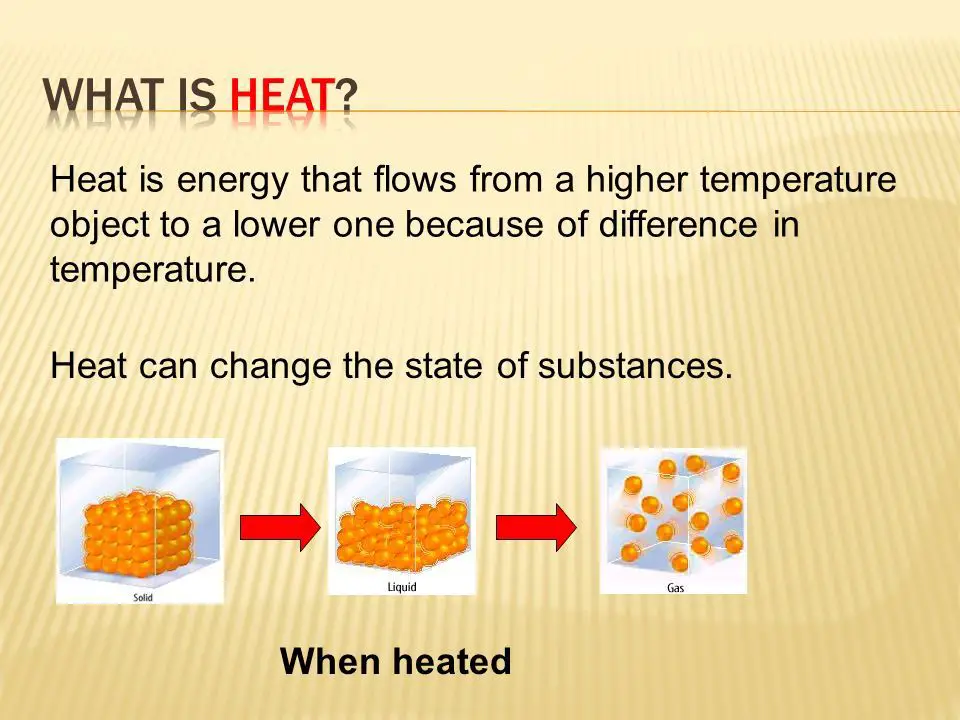
Heat flows naturally from higher temperature objects to lower temperature objects. If you place a hot cup of coffee next to a cold glass of water on a table, the coffee will transfer some of its heat to the water glass until they reach the same temperature. This is because heat moves spontaneously down a thermal gradient, from areas of high temperature to areas of low temperature.
When two objects are in contact, heat will flow between them until they reach the same temperature. At this point, they are in thermal equilibrium and no net heat transfer occurs. The colder object gains energy and increases in temperature, while the hotter object loses energy and decreases in temperature. Once at the same temperature, the rate of heat gain equals the rate of heat loss between the objects. Thermal equilibrium is the state where there is no net heat flow between objects because their temperatures have equalized.
Heat is fundamentally related to thermodynamics, which describes the flow and conversion of energy. The laws of thermodynamics explain key concepts about heat and its relationship to energy.
Laws of thermodynamics
The laws of thermodynamics govern how heat and energy interact in physical systems. They were developed during the Industrial Revolution to optimize early steam engines and have become fundamental concepts in physics. There are three main laws of thermodynamics:
First law – The law of conservation of energy states that energy cannot be created or destroyed, only converted from one form to another. This means the total energy in a closed system always remains constant. Heat transfer is an energy conversion process, so the energy gained or lost through heating and cooling must equal zero. This is why heat and energy are so closely linked.
Second law – The law of increasing entropy states that in any closed system, the amount of disorder, or entropy, always increases over time. This means that concentrated heat energy naturally spreads out and becomes more disordered. Heat transfer is an entropy-increasing process, as heat dissipates from warmer objects to cooler surroundings. This is why hot coffee left on a table eventually cools down to room temperature.
The laws of thermodynamics govern heat transfer processes and explain why heat behaves the way it does. They show that while energy is conserved, entropy inevitably grows through the dispersal of heat. These fundamental laws connect heat, energy, and order in physical systems.
Real-world applications of heat
Heat plays a crucial role in various real-world applications and systems that we use every day. Some of the most notable examples include:
Heating and cooling systems – Heat pumps and air conditioners use principles of heat transfer to provide heating, ventilation, and air conditioning (HVAC) in buildings. Heat pumps move heat from a cold space to a warm space for heating, or vice versa for cooling. Modern HVAC systems manipulate refrigeration cycles and heat exchangers to control indoor environments.
Cooking equipment – Appliances like stoves, ovens, grills, and toasters all rely on heat transfer to cook food. The heating elements in these appliances convert electricity into heat which is then transferred to the food via conduction and convection. Different cooking techniques use various combinations of heat transfer.
Engines – Internal combustion engines found in most vehicles generate heat from the combustion of fuel. This heat and thermal energy is converted into mechanical work that powers the engine. Engines also rely on cooling and lubrication systems to regulate heat transfer and prevent overheating.
Chemical processes – Many industrial chemical processes involve the application or removal of heat to drive chemical reactions. Exothermic reactions that generate heat may require cooling, while endothermic reactions may require heating. Careful control of heat transfer is needed for safety and efficiency.
Heat transfer in nature
Heat moves through the atmosphere and oceans in complex ways that strongly influence Earth’s climate and weather. The sun is the primary source of heat that warms the atmosphere and ocean. Solar radiation enters the atmosphere and is absorbed by the land and oceans. The oceans can store large amounts of heat energy due to water’s high heat capacity. The movement of ocean currents redistribute heat around the globe.
Heat transfer is critical for thermoregulation in animals. Mammals and birds are endotherms, meaning they generate their own body heat metabolically. They use mechanisms like panting, sweating, shivering, and vasodilation to help maintain a stable internal body temperature despite changing external temperatures. Other animals like reptiles and fish are ectotherms whose body temperature varies with their external environment. Many ectotherms use behavioral adaptations like basking and seeking shade to regulate body temperature.
History and Pioneers
The study of heat has a long history, with many key experiments and discoveries that shaped our modern understanding. Some of the most notable pioneers include:
Joseph Fourier – In the early 1800s, Fourier studied heat transfer and analyzed the flow of heat between bodies. He described the idea of thermal conductivity and developed Fourier’s Law relating heat flux to temperature gradient. His work laid the foundations for the field of heat conduction.
James Prescott Joule – Joule established the mechanical equivalent of heat in the 1840s, showing that heat is a form of energy. His famous paddle wheel experiment demonstrated that mechanical work can be converted to heat with a predictable ratio.
Rudolf Clausius – In the 1850s, Clausius coined the term “entropy” and developed the Second Law of Thermodynamics. His work with heat engines and refrigerators advanced thermodynamics.
Ludwig Boltzmann – In the late 1800s, Boltzmann did pivotal work relating temperature to molecular motion and statistical mechanics. He helped explain the behavior of heat at the microscopic level.
These pioneering scientists helped establish that heat is a form of kinetic energy related to molecular motion. Their discoveries created the foundations of thermodynamics and heat transfer theory.