How Does Thermal Energy Relate To Heat Quizlet?
Thermal energy refers to the total energy of microscopic motion and vibration of particles in an object, which manifests itself as heat. Heat is the transfer of thermal energy from one object or system to another due to temperature differences. Thermal energy is essentially the internal energy present in matter in the form of kinetic energy and potential energy at the molecular level. Heat is the flow of thermal energy driven by entropy between two systems in thermal contact. All matter has thermal energy, but heat only transfers between objects or systems with differing temperatures.
Thermal energy is often used interchangeably with heat, but they are distinct concepts. Thermal energy exists in the atoms and molecules that make up matter, relating to the kinetic and potential energy of the particles. Heat deals with the transfer of that internal energy between two systems based on temperature gradients and thermodynamic principles. So while thermal energy exists in an isolated system, heat requires at least two systems to be exchanged.
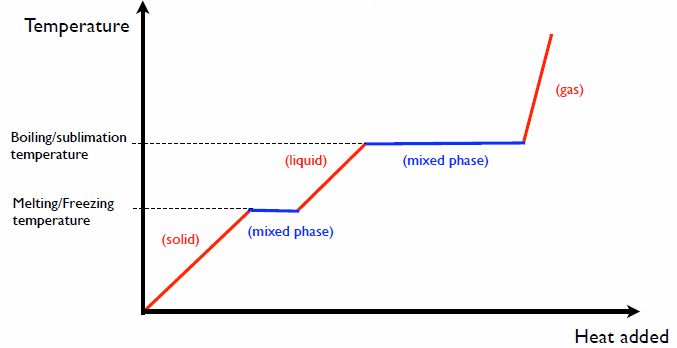
Thermal energy can change the physical properties of matter through transfers of heat. Understanding the relationship between thermal energy and heat is key for thermodynamics and the efficient transfer, conversion and utilization of energy in various processes and systems.
Forms of thermal energy transfer
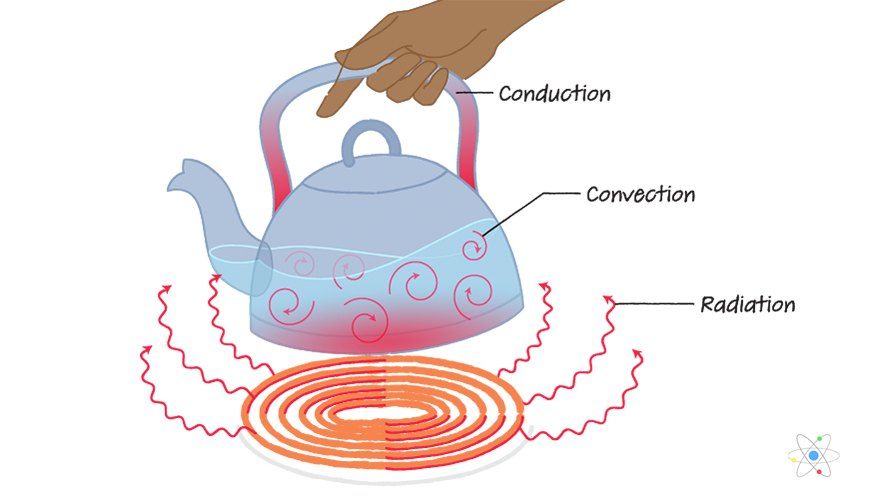
There are three main ways that thermal energy can be transferred between objects or locations: conduction, convection, and radiation.
Conduction
Conduction is the transfer of thermal energy between objects that are in direct physical contact with each other. Thermal energy flows from the object at a higher temperature to the object at a lower temperature. The rate of conductive heat transfer depends on the temperature difference between the objects and the conductive properties of the materials involved.
Convection
Convection is the transfer of thermal energy by the movement of liquids and gases. As a liquid or gas gains thermal energy, it becomes less dense and rises. Colder, denser liquid or gas then moves to take its place, creating a continuous circulation. Examples of convection include heating air currents and ocean currents.
Radiation
Radiation is the transfer of thermal energy by electromagnetic waves directly between objects, without requiring direct contact or a transport medium. All objects emit and absorb thermal radiation depending on their temperature. Net heat transfer occurs from higher temperature to lower temperature objects.
Examples of conduction
Conduction is the transfer of heat energy between particles that are directly touching each other. Heat conduction commonly occurs in solids, where atoms and molecules are in constant contact. Here are some examples of heat conduction through solids:
- Touching a hot stove – the metal pan conducts heat quickly to your hand
- Wrapping your hands around a hot mug of coffee to warm them up
- Using a metal spoon to stir a hot pot of soup transfers heat to the spoon
- Heat sinks on computers use metal with high conductivity like aluminum to dissipate heat from the CPU
- Insulating homes by adding fiberglass in walls and attics reduces conduction from the hot exterior to the cooler interior
- Diamonds conduct heat away from the tip of cutting tools allowing them to stay sharp longer
In each example, heat is directly transferred through materials touching each other. The rate of conduction depends on the conductivity of the material and the temperature difference driving the flow of heat.
Examples of convection
Convection is the transfer of heat through the movement of fluids, including both gases and liquids. Hotter fluids rise and cooler fluids fall under the influence of gravity and density differences. Here are some examples of convection:
-
Hot air balloons rise because the air inside the balloon is heated by the burner, making it less dense than the cooler air outside the balloon. The hotter, less dense air rises through the cooler, denser outside air.
-
The circulation of air due to room heaters, central heating systems, or the sun heating up air near the ground, which then rises while cool air drops down to take its place.
-
Convection currents in water such as those that contribute to ocean currents and sea breezes.
-
Convection ovens and stoves that rely on fans or natural air circulation to transfer heat around food more effectively than regular ovens.
In each example, the fluid moves to transfer heat energy from one area to another based on differences in temperature and density.
Examples of radiation
Radiation is the transfer of thermal energy through electromagnetic waves or photons. Unlike conduction and convection, radiation does not require direct contact or a transport medium like air or water. Some common examples of radiation transferring thermal energy in everyday life include:
– The sun warming the Earth through solar radiation
– Heat from a fire felt at a distance as infrared radiation
– Microwave ovens heating food via microwave radiation
– An incandescent light bulb producing visible light and infrared radiation
– Radiant floor heating systems transferring heat through a floor’s surface
– The Earth releasing thermal radiation into space, enabling Earth’s heat balance
– Infrared cameras detecting thermal radiation emitted by objects
In each example, thermal energy is transferred through electromagnetic waves that travel directly from a hotter object to a colder one without warming the space between them. Understanding radiation is crucial to applications like solar heating, remote temperature measurements, and thermal imaging.
Measuring Thermal Energy
Thermal energy cannot be measured directly, but there are several quantities related to thermal energy that can be measured. The most common ways of measuring thermal energy are through temperature, heat capacity, and calorimeters.
Temperature is a measure of the average kinetic energy of particles in a substance. Temperature is measured in units like degrees Celsius, Fahrenheit, or Kelvin. Thermometers use various properties like volume expansion to measure temperature. Higher temperatures mean particles have greater average kinetic energy.
Heat capacity is the amount of energy needed to raise a substance’s temperature by 1 degree. Substances with higher heat capacities require more energy to change temperature. Heat capacity depends on the amount, type, and mass of particles in a substance. Calorimeters can be used to experimentally determine heat capacity.
Calorimeters allow measuring the energy transferred as heat in chemical reactions or phase changes. By measuring temperature change in a known mass of water, calorimeters calculate the amount of heat energy absorbed or released. Calorimetry provides quantitative data about a substance’s thermal energy and heat capacity.
Thermal Energy Applications
Thermal energy has many important real-world applications. Three major areas that rely on thermal energy are engines, heating/cooling, and cooking.
In engines such as car engines, thermal energy is transformed into mechanical energy that powers the vehicle. Fuel is burned inside the engine, releasing thermal energy that causes the pistons to move up and down. This kinetic energy is then transferred to the wheels to propel the car forward.
Heating and cooling systems also depend on thermal energy transfer. Furnaces and boilers use the combustion of fuel to heat up air or water, which is then distributed throughout a building via vents or pipes. Air conditioners and refrigerators move thermal energy from a cool space into a hot external environment using compressed refrigerants and a compressor.
Cooking relies on multiple forms of thermal energy transfer. Pots and pans conduction heat from the stove to cook food. Ovens use thermal radiation to bake or roast food. Convection ovens have fans that circulate hot air around food. Microwave ovens use electromagnetic radiation to vibrate water molecules in food, producing internal heat through friction.
So whether it’s powering vehicles, keeping buildings at comfortable temperatures, or preparing meals, thermal energy is a vital part of our everyday lives.
Thermal Energy and Work
The first law of thermodynamics describes the relationship between thermal energy and work. It states that the change in internal energy of a system is equal to the amount of heat supplied to the system, minus the amount of work done by the system on its surroundings.
Thermal energy can be converted into work. For example, in a steam engine, the thermal energy from burning fuel is used to convert water into high pressure steam. This steam is then used to push a piston and do mechanical work. The thermal energy is converted into the mechanical work delivered by the piston.
Work can also be converted into thermal energy. For example, when a moving piston is brought to rest by friction, its kinetic energy is converted to thermal energy in the form of heat. The faster the piston is moving, the more heat is produced when it is stopped by friction.
According to the first law, the amount of thermal energy gained or lost by a system equals the amount of work done on or by the system. This means that if a system gains thermal energy, it can do more work. Likewise, a system that loses thermal energy has less energy available to do work.
The first law of thermodynamics describes the quantitative relationship between heat, work and internal energy. It shows that thermal energy and mechanical work are two forms of energy transfer that can be converted into one another.
Quizlet thermal energy review
Quizlet can be a helpful tool for reviewing key concepts related to thermal energy and heat. Here is a summary of some important points, along with potential quiz questions and answers:
Summary
Thermal energy refers to the total kinetic and potential energy of all the molecules within an object. Heat is the transfer of thermal energy from one object or system to another due to a temperature difference. The main methods of heat transfer are conduction, convection and radiation.
Conduction is the transfer of heat through direct contact. Metals are good conductors. Convection is the transfer of heat through the movement of fluids or gases. Heated air rising is an example. Radiation is the transfer of heat not requiring a medium to travel through. Infrared radiation from the sun is an example.
Temperature measures the average kinetic energy of molecules. Thermal energy always flows from higher temperature to lower temperature. Insulators resist heat flow while conductors allow heat flow more easily.
Quiz Questions
Q: What is the difference between heat and temperature?
A: Heat is the transfer of thermal energy from one object or system to another due to a temperature difference. Temperature measures the average kinetic energy of molecules.
Q: What are the three main methods of heat transfer?
A: The three main methods of heat transfer are conduction, convection and radiation.
Q: True or false: Metals are good insulators.
A: False. Metals are good conductors of thermal energy.
Q: What causes convection currents in air, water and other fluids?
A: Convection currents are caused by density differences resulting from temperature differences in the fluid.
Q: Why does heat rise?
A: Hot air is less dense than the surrounding cooler air, so it rises due to buoyancy.
Answers
Heat transfer, thermal energy, temperature, conduction, convection, radiation, conductors, insulators, density differences, buoyancy.
Conclusion
In summary, thermal energy refers to the total kinetic energy of molecules within a substance, which relates to the temperature and motion of those molecules. Heat is the transfer of thermal energy from one object or system to another due to temperature differences. We explored three main forms of heat transfer – conduction, convection and radiation. Understanding how thermal energy transfers as heat is key for many applications like insulation, engines, thermodynamics and more. Through examples and descriptions, this guide reviewed core concepts around how thermal energy relates to heat.
To recap, conduction involves direct contact transferring heat between two objects, convection relies on the movement of fluids to transfer heat, and radiation works through electromagnetic waves emitting thermal energy. Measuring thermal energy requires an understanding of systems, heat transfer and thermodynamic principles. Engineers apply these principles to improve designs and optimize thermal efficiency. After reading this guide, you should have a foundational grasp of the relationship between thermal energy and heat.