How Does Energy Get Transferred?
Energy transfer refers to the process of energy moving from one object or system to another. Some basic examples of energy transfer include:
- Heat flowing from a hot stovetop into a pot of water, causing the water to boil.
- Light energy from the sun being absorbed by solar panels, which then convert it into electrical energy.
- The chemical energy stored in gasoline moving into the engine of a car when the gasoline is burned, powering the engine.
- Electrical energy moving through power lines into homes and businesses to power lights, appliances, and devices.
In all these examples, energy is transferred from one place to another in order to make some kind of change or do work. The study of energy transfer seeks to understand how these transfers occur on a molecular level and how energy can be directed in useful ways.
Forms of Energy
There are many different forms that energy can take. Some of the main forms of energy include:
Potential energy – This is energy that is stored and held in readiness. Some examples are the energy stored in water held behind a dam or energy stored in a spring that is compressed. The energy is released when the water is allowed to flow or the spring expands.
Kinetic energy – This is the energy of motion. A rolling ball or a speeding car both have kinetic energy.
Thermal energy – Often called heat, thermal energy relates to the motion of atoms and molecules. As an object is heated up, its atoms and molecules move and vibrate faster, increasing its thermal energy.
Radiant energy – This is electromagnetic energy that travels in transverse waves. Light from the sun is an example of radiant energy.
Electrical energy – The movement of electrons creates electrical energy. This energy can be generated and stored for later use.
Chemical energy – Energy stored in the bonds between atoms and molecules. Food and batteries both store chemical energy that is released during chemical reactions.
Nuclear energy – The energy stored within the nucleus of an atom, released through nuclear fission or fusion.
Energy Transfer Methods
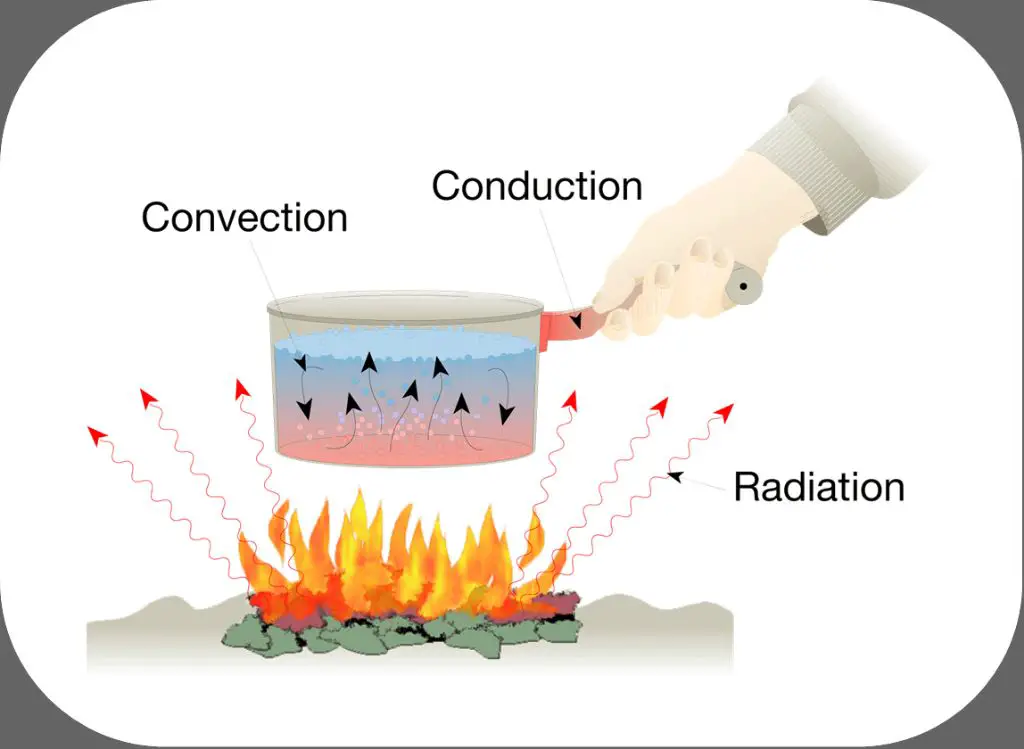
Energy can be transferred between objects or locations through various methods. Some of the main methods of energy transfer are conduction, convection, radiation, mechanical waves, electrical current, and chemical reactions.
Conduction is the transfer of energy between objects that are in direct physical contact. It involves the transfer of kinetic energy between molecules when they collide. Metals are good conductors of thermal energy.
Convection is the transfer of energy in a fluid through the circulation or movement of the fluid itself. It transfers heat energy through liquids and gases. Examples are hot air rising or water currents distributing heat in the oceans.
Radiation is the transfer of energy by electromagnetic waves or photons. No direct contact is required between the objects exchanging energy. The sun transfers warmth to the Earth through radiation.
Mechanical waves like sound can transmit energy as pressure waves through a medium like air or water without actually moving matter from one location to another.
An electrical current involves the movement of charged particles which can transfer energy. Charged particles like electrons can move through wires and circuits, facilitating energy transfer.
In a chemical reaction, energy is transferred when chemical bonds are broken and new bonds are formed. Reactions like combustion transfer large amounts of energy in the form of heat and light.
Conduction
Conduction is the transfer of energy between objects that are in direct physical contact with each other. It occurs when heat is transferred through a medium, such as a solid, liquid or gas. Conduction requires direct contact between molecules of the conducting medium. It is most effective in solids since their molecules are closer together compared to liquids or gases.
For example, when you place a metal spoon in a hot cup of soup, the spoon quickly becomes hot as thermal energy is conducted from the soup through the spoon. The spoon’s molecules gain kinetic energy as they collide with the energetic soup molecules. This chain reaction spreads the thermal energy along the spoon from the point of contact. The denser the medium, the easier it is for the thermal energy transfer since the molecules are closer together.
Conduction can flow in different directions based on the temperature gradient. Heat will always transfer from regions of higher temperature to regions of lower temperature. For instance, if you grab the opposite end of the spoon sticking out of the hot soup, the thermal energy will be conducted from your hand into the cooler spoon. This demonstrates how conduction enables the redistribution of thermal energy from hotter to cooler areas until thermal equilibrium is reached.
Convection
Convection is the transfer of energy by currents or airflow. It involves the movement of liquids and gases that carry thermal energy from one place to another. As the liquid or gas is heated, it expands and becomes less dense, allowing it to rise while cooler, denser liquid or gas sinks and replaces it. This creates currents and airflows that distribute and circulate heat, creating an effective method of energy transfer.
Convection occurs commonly in nature through wind currents and ocean currents. It also occurs in engineered systems like heating and cooling systems that use fans or pumps to circulate air or water. As the fluid moves, it carries energy from hot surfaces to cooler surfaces, allowing for the uniform heating or cooling of a space. Convection takes advantage of the motion of liquids and gases to efficiently transfer thermal energy.
Radiation
Radiation is the transfer of energy by electromagnetic waves. Unlike conduction and convection, which require matter to transfer energy, radiation can transfer energy across empty space. This is because radiation does not rely on particle collisions, but rather electromagnetic waves. These waves can travel through a vacuum at the speed of light.
Examples of radiation include visible light from the sun, radio waves, microwaves, infrared radiation, ultraviolet radiation, x-rays, and gamma rays. All these types of radiation are part of the electromagnetic spectrum, which includes waves of all frequencies. When these waves strike matter, like our skin, they impart their energy to atoms and molecules, effectively transferring energy. This allows the sun’s energy to reach us across the vacuum of space.
Radiation does not require a medium to transfer energy. While conductive and convective energy transfer rely on interactions between particles in matter, radiative transfer can occur across regions empty of matter. This property makes radiation uniquely capable of transferring energy over long distances where conduction and convection are ineffective.
Mechanical Waves
Mechanical waves transfer energy through the vibration of a medium such as a solid, liquid or gas. The vibration causes the particles that make up the medium to oscillate, transferring energy from one particle to the next in a chain reaction. Unlike electromagnetic waves like light which can travel through a vacuum, mechanical waves require a medium to propagate.
One common example of a mechanical wave is sound. When an object vibrates, it sets the particles in the surrounding air into vibrational motion. The vibrating air particles exert a force on adjacent particles, transferring the energy outward in a wave. As the vibration ripples outwards, it eventually reaches our ears, enabling us to perceive the sound. Other examples of mechanical waves include waves in bodies of water, seismic waves from earthquakes traveling through the ground, and waves traveling along ropes and springs.
Electrical Current
Electrical current is one of the key ways energy is transferred. It involves the flow of electrons through a conductor, like a metal wire. The electrons are negatively charged particles that migrate from one atom to another in the conductor. This electron flow is what provides power and enables electrical devices to operate.
When there is a high concentration of electrons at one end of the conductor and a low concentration at the other end, it creates a force that pushes the electrons to flow from the high to the low concentration. Materials like copper that have a crystalline structure make it easier for electrons to move freely. The greater the push of electrons, measured in voltage, the faster the rate of flow, measured in amperage.
Electrical current transfers energy in several ways. It converts electrical energy into other forms like light, heat, or motion to power devices. It also enables electrical signals that carry information. Overall, the motion of electrons through conductors is a key means of distributing energy to meet many human needs.
Chemical Reactions
One of the main ways that energy gets transferred is through chemical reactions. During chemical reactions, the chemical bonds between atoms and molecules are broken and formed. Breaking chemical bonds requires energy input, while forming bonds releases energy. The energy changes associated with breaking and forming bonds are characteristic for each reaction.
For example, when gasoline and oxygen react in combustion, the chemical bonds in the gasoline molecules are broken, requiring energy input. However, new bonds are formed between the atoms of the combustion products, carbon dioxide and water. The formation of these new bonds releases more energy than was required to break the bonds in the gasoline. The release of excess energy in this exothermic reaction provides the energy that drives car engines.
The difference between the energy required to break bonds and the energy released when new bonds form determines whether a chemical reaction absorbs or releases energy overall. Exothermic reactions like combustion release net energy, while endothermic reactions require net energy input. The transfer of energy through breaking and forming chemical bonds drives many important processes, from energy production to cooking food.
Energy Conversion
Energy can be converted from one form to another. This allows us to transform energy into more useful forms and harness it to power human activities. Some common energy conversions include:
Chemical to Thermal: Burning fossil fuels like coal, oil, and natural gas converts the chemical energy stored in their molecular bonds into heat energy. This thermal energy can be used for heating or converted into mechanical energy to drive turbines and generate electricity.
Solar to Thermal: Solar panels convert sunlight into heat energy that is used to warm water. The thermal energy absorbed from the sun’s rays gets transferred into hot water.
Mechanical to Electrical: Turbines in hydroelectric dams use the mechanical energy of moving water to spin rotors and generate electrical current via electromagnetic induction.
Chemical to Electrical: Fuel cells convert the chemical potential energy of hydrogen into electricity through electrochemical reactions.
These are just a few examples. Energy conversion enables us to transform energy from forms that are difficult to use into more useful forms that power society. Understanding these conversions allows engineers to better tap into energy sources and meet human needs.