How Do You Calculate Change In Energy In Physics?
Energy is one of the most fundamental concepts in physics. Energy is defined as the ability to do work or produce heat. The idea that energy cannot be created or destroyed is known as the law of conservation of energy. This law states that the total energy in an isolated system remains constant. While energy can change from one form to another, the total amount of energy does not change.
Being able to calculate changes in energy is critical for analyzing many physics systems and processes. Knowing how to precisely determine the change in energy provides great insight into the behavior of objects and systems. Calculating energy transfers and transformations helps reveal how different types of energy interact and influence outcomes.
This article will explain the methods for calculating changes in the main forms of energy encountered in physics. We will cover calculating kinetic and potential energy changes as well as heat and work transfers. Examples will be provided to demonstrate the principles. Through a solid understanding of energy change calculations, many physical processes can be quantitatively evaluated and understood.
Definition of Energy
In physics, energy is defined as the ability to do work or produce heat. Energy exists in various forms such as kinetic energy, potential energy, thermal energy, electromagnetic radiation, sound energy, elastic energy, nuclear energy, and so on. These forms of energy can be grouped into two main categories:
- Kinetic energy – the energy possessed by an object due to its motion. For example, the kinetic energy of a moving car or a flying ball.
- Potential energy – stored energy possessed by an object due to its position or configuration. For example, the potential energy of a compressed spring, water held behind a dam, or a book placed on a shelf.
The law of conservation of energy states that the total energy in an isolated system remains constant. Energy can only be transformed from one form into another or transferred between objects, but it cannot be created or destroyed. Calculating the change in energy is a common application of the law of conservation of energy in physics problems.
Law of Conservation of Energy
The law of conservation of energy states that the total energy in an isolated system remains constant. Energy cannot be created or destroyed, only converted from one form to another. This means that the amount of energy in a system at the beginning must equal the amount of energy at the end, regardless of any changes that take place in between.
For example, consider a ball rolling down a hill. At the top of the hill, the ball has potential energy due to its position in the gravitational field. As the ball rolls down the hill, this potential energy gets converted into kinetic energy, the energy of motion. Even though the forms of energy change, the total amount of energy in the system (ball + Earth) remains the same.
The law of conservation of energy is an absolute law that holds in all situations. It allows us to track changes and make calculations involving energy transfers and transformations. By accounting for all the various forms energy can take, we can equate the total energy at the beginning and end of any process and thereby analyze and quantify energy changes in a system.
Types of Energy Changes
Energy can change in several ways during an interaction or process. Some common types of energy changes include:
Heat (Q) – Heat is energy transferred between objects or systems due to a temperature difference. For example, when you boil water on a stove, the burner provides heat that raises the temperature of the water.
Work (W) – Work is energy transferred when a force causes an object or system to move. For example, when you lift a book from the floor to a table, your arms do work to move the book upwards against gravity.
Chemical Energy – Chemical energy is energy stored in the bonds between atoms and molecules. Chemical reactions involve breaking and forming of chemical bonds and can release or absorb heat. For example, burning gasoline involves breaking chemical bonds which releases heat and does work.
Nuclear Energy – Nuclear energy comes from changes within atomic nuclei, such as nuclear decay, nuclear fusion, or nuclear fission. Nuclear reactions can release very large amounts of heat and radiation. For example, nuclear power plants split uranium atoms to generate heat and electricity.
Electrical Energy – Electrical energy results from the flow of charges. For example, energy is transferred to run a lightbulb when charges flow through the wires and bulb. Batteries also store chemical energy and convert it to electrical energy.
Sound Energy – Sound is a vibration that travels as a wave through matter like air and water. It carries energy transmitted by a sound source. Louder sounds have greater intensity and more energy.
Light Energy – Light is a type of radiation that can travel through a vacuum as electromagnetic waves. Light sources like the sun transfer energy. When light is absorbed by matter, it is transformed into thermal energy and does work.
Calculating Kinetic Energy Changes
Kinetic energy is the energy of motion. The kinetic energy of an object depends on its mass and velocity. The formula for kinetic energy is:
KE = 1/2 * m * v^2
Where:
- KE is the kinetic energy in joules (J)
- m is the mass of the object in kilograms (kg)
- v is the velocity of the object in meters per second (m/s)
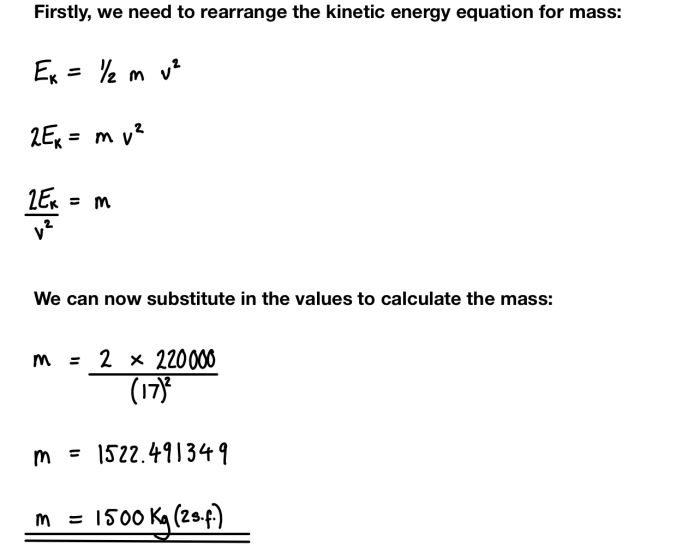
To calculate the change in kinetic energy of an object, you can use the kinetic energy formula to calculate the initial and final kinetic energies and take the difference. For example:
An object with a mass of 2 kg accelerates from 5 m/s to 10 m/s. Calculate the change in kinetic energy.
Initial KE = 1/2 x 2 x 5^2 = 25 J
Final KE = 1/2 x 2 x 10^2 = 100 J
Change in KE = Final KE – Initial KE
= 100 J – 25 J
= 75 J
Therefore, the change in kinetic energy is 75 J. By calculating the kinetic energy before and after a change using the formula, you can determine the difference to find the change in kinetic energy.
Calculating Potential Energy Changes
Potential energy is the stored energy an object has due to its position or state. There are several types of potential energy to consider in physics:
- Gravitational Potential Energy – Depends on an object’s mass, gravity, and height above a reference point.
- Elastic Potential Energy – Depends on the spring constant of a spring and how far it’s stretched or compressed.
- Chemical Potential Energy – Depends on the types of molecules and chemical bonds in a substance.
To calculate changes in potential energy, you need to use the appropriate potential energy equation and determine the energy at the initial and final state. Then take the difference to find the change.
For gravitational potential energy, the equation is:
GPE = mgh
Where m is mass, g is the gravity constant (9.8 m/s2 on Earth), and h is height. So if you know the mass, gravity, and change in height, you can calculate the change in gravitational potential energy.
For example, if a 2kg box was lifted from the ground up to a 3m high shelf, the change in GPE would be:
Final GPE = mgh
= (2kg)(9.8m/s2)(3m)
= 58.8 J
Initial GPE = 0 J (started on the ground)
Change in GPE = Final – Initial
= 58.8 J – 0 J
= 58.8 J
To calculate elastic potential energy changes, use the equation:
EPE = 1/2 kx2
Where k is the spring constant and x is the compression or stretch distance. Calculate the initial and final EPE based on the spring deformation, then take the difference.
Understanding these potential energy formulas and how to apply them is key to determining energy changes in physics problems.
Calculating Heat and Work
Heat transfer and work done are two ways that energy can be transferred between a system and its surroundings. To calculate how these processes impact the energy of a system, we need to understand how they are defined and quantified.
Heat transfer refers to the energy transfer that occurs between objects due to a temperature difference. It flows spontaneously from higher temperature to lower temperature objects. The amount of heat transfer can be calculated using the formula:
Q = mcΔT
Where Q is the heat transfer, m is the mass of the object, c is the specific heat capacity, and ΔT is the change in temperature. The specific heat tells us how much energy is required to change the temperature of 1 kg of a substance by 1°C. By multiplying it by the mass and temperature change, we can find the energy gained or lost due to heat transfer.
Work is energy transfer that occurs when a force displaces an object. It is calculated as:
W = Fd
Where W is work, F is force, and d is displacement. By multiplying the force applied by the distance moved, we quantify the work performed on or by the object.
Both heat and work can lead to changes in the mechanical energy of a system. Gaining heat energy will increase the kinetic and potential energy, while losing heat decreases it. Work done on a system increases its mechanical energy, while work done by the system decreases its energy. By carefully accounting for heat transfer and work, we can apply the law of conservation of energy to analyze a wide range of physics problems.
Conservation of Mechanical Energy
The law of conservation of mechanical energy states that in a closed system with no nonconservative forces, the total mechanical energy remains constant. Mechanical energy is the sum of potential energy and kinetic energy. This law can be used to calculate energy changes in various scenarios.
For example, consider a ball being dropped from a height h above the ground. Using conservation of mechanical energy:
Initial energy = Final energy
mgh (potential energy) = 1/2mv^2 (kinetic energy)
Where m is mass, g is gravitational acceleration, h is height, and v is velocity.
We can use this equation to calculate the velocity or height, if the other variables are known. This allows us to quantify the exchange between potential and kinetic energy for the ball during its fall.
As another example, imagine a roller coaster car starting at rest on top of a hill of height h. As it descends, it accelerates due to gravity. At the bottom, it has velocity v. Using conservation of mechanical energy:
mgh = 1/2mv^2
We can calculate the velocity v knowing the initial height h. This demonstrates that the potential energy is converted into kinetic energy for the roller coaster car.
In both cases, mechanical energy is conserved as the total remains constant. Calculating the potential and kinetic energies at different points allows us to analyze how energy is transferred between different forms while obeying the conservation law.
Power and Efficiency
Power is defined as the rate at which work is done or energy is transferred. It is calculated by dividing the amount of work done or energy transferred by the time taken. The units of power are joules/second or watts.
Efficiency describes how much useful energy or work output you get from an energy input. It is calculated by dividing the useful energy or work output by the total energy input. Efficiency is expressed as a percentage or decimal.
Power and efficiency are related to energy changes over time. The more power a system has, the faster it can do work or transfer energy. Higher efficiency means more of the input energy is converted to useful output energy, rather than being lost or wasted.
For example, a 100 Watt light bulb converts 100 Joules of electrical energy to light and heat energy every second. If the light bulb is 10% efficient, then 10 Joules of light energy is produced each second, while 90 Joules is lost as heat. More efficient light bulbs can convert a greater percentage of input energy to useful light energy.
When analyzing energy changes in physics, power and efficiency help describe the rate and extent of energy transfers and transformations. Calculating power and efficiency provides insights into how quickly and effectively a system can do work or change energy forms over time.
Practice Examples
To demonstrate how to calculate different types of energy changes, let’s go through some example physics problems:
Example 1: A 0.5 kg ball is dropped from a height of 10 m. Calculate the change in gravitational potential energy of the ball.
Solution: Gravitational potential energy depends on mass, gravity, and height. The equation is:
ΔPEgrav = mgh
Where m is mass (0.5 kg), g is gravity (9.8 m/s2), and h is height (10 m)
Plugging in the values:
ΔPEgrav = (0.5 kg)(9.8 m/s2)(10 m)
ΔPEgrav = 49 J
The gravitational potential energy decreased by 49 J when the ball was dropped.
Example 2: A car accelerates from 5 m/s to 25 m/s over 10 seconds. Calculate the change in kinetic energy.
Solution: Kinetic energy depends on mass and velocity. The equation is:
ΔKE = 1/2mv2 – 1/2mv1
Where m is mass, v2 is final velocity, and v1 is initial velocity. Let’s assume the mass is 1000 kg.
v1 = 5 m/s
v2 = 25 m/s
Plugging in:
ΔKE = 1/2(1000 kg)(25 m/s)2 – 1/2(1000 kg)(5 m/s)2
ΔKE = 312,500 J – 12,500 J
ΔKE = 300,000 J
The car’s kinetic energy increased by 300,000 J.