Can Atoms Be Converted Into Energy?
Introducing Atomic Energy
Atoms are the fundamental building blocks of matter. They consist of a dense nucleus containing protons and neutrons, surrounded by a cloud of orbiting electrons. The number of protons defines an atom’s chemical identity and atomic number. Atoms of the same element have the same number of protons but can have different numbers of neutrons, resulting in different isotopes of that element.
Nuclear energy originates from the forces that hold the atomic nucleus together. Directing the processes that release this energy, such as nuclear fission or fusion, produces massive amounts of heat which can be converted into electricity. Nuclear reactions convert a small amount of mass into a large amount of energy, in accordance with Einstein’s famous equation E=mc^2.
This article will examine how atoms can be utilized for energy production via nuclear processes. We will explore the physics behind fission and fusion, and discuss how nuclear power plants are able to harness atomic energy for practical use.
History of Nuclear Physics
The controlled use of nuclear energy first became possible due to key discoveries in physics in the late 19th and early 20th centuries. In 1896, French physicist Henri Becquerel accidentally discovered radioactivity while doing experiments with uranium salts. He went on to show that the radiation emitted by uranium and other radioactive elements differed from X-rays discovered by Wilhelm Roentgen the year before.
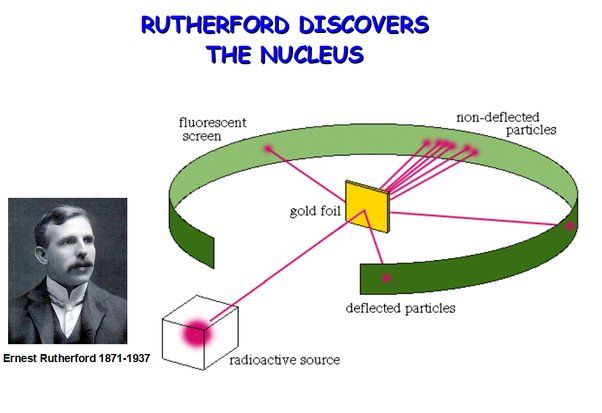
Further groundbreaking work was done by Ernest Rutherford, who conducted experiments between 1908 and 1913 that led to a revolutionary new model of the atom, with a dense positively-charged nucleus surrounded by orbiting negatively-charged electrons. Rutherford recognized the enormous energy contained within the atomic nucleus.
The equation E=mc2, proposed by Albert Einstein in 1905, demonstrated that mass and energy are interchangeable. This revealed the vast amounts of energy that could be released by converting small amounts of mass. This groundbreaking concept eventually enabled both nuclear fission and fusion processes to be unlocked.
These key discoveries in physics laid the foundation for the controlled use of nuclear energy through fission and fusion, by revealing the composition of the atom and vast untapped energy within its nucleus.
Nuclear Fission
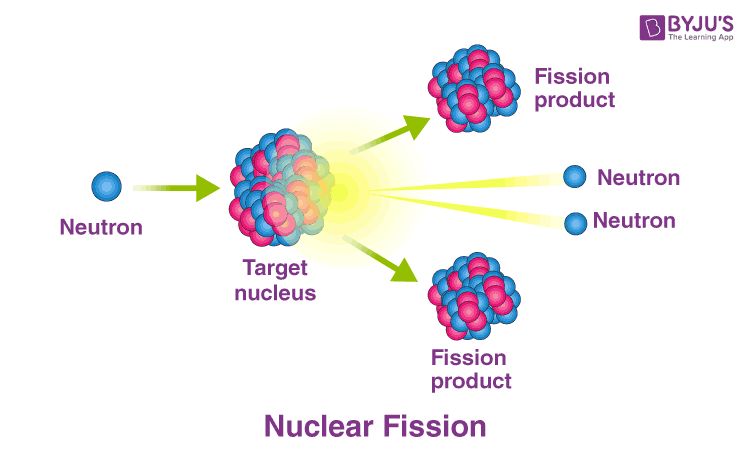
Nuclear fission is the process by which a heavy atomic nucleus, such as uranium or plutonium, splits into two lighter nuclei. This process releases energy as neutrons and photons. In nuclear fission, a neutron collides with a heavy nucleus and is absorbed. This collision destabilizes the nucleus and causes it to split into two smaller nuclei, called fission fragments, and release two or three additional neutrons. The fission of uranium-235, for example, most often splits the nucleus into krypton-92 and barium-141. These fission fragments have a lower binding energy than the original uranium nucleus, meaning that the strong nuclear force holding them together is less. This difference in binding energy gets converted into kinetic energy of the fission fragments, emitting energy equivalent to about 200 MeV.
Nuclear fission is used to generate electricity in nuclear power plants. Uranium or plutonium isotopes are concentrated enough to undergo fission chain reactions inside a nuclear reactor core. The chain reaction is controlled by control rods that absorb excess neutrons. The enormous heat generated by fission is used to boil water into steam. This steam then spins turbines to generate electricity. Nuclear fission generates around 10-11% of the world’s electricity. However, concerns around waste disposal, accidents, and weapons proliferation remain challenges for civilian nuclear power.
Nuclear Fusion
Nuclear fusion is a reaction in which two or more atomic nuclei collide at very high speeds and join to form a new type of atomic nucleus. During this process, a small amount of mass is lost and converted into a massive amount of energy according to Einstein’s equation E=mc2. Nuclear fusion is the source of the sun’s energy as hydrogen atoms fuse together under extreme heat and pressure to form helium, releasing energy in the process. This is the opposite reaction of nuclear fission (splitting of atoms).
Nuclear fusion can also take place in experimental fusion reactors here on Earth. One approach is magnetic confinement, which uses strong magnetic fields to contain an extremely hot plasma of hydrogen isotopes. As the plasma reaches extreme temperatures of 150 million degrees Celsius, the hydrogen nuclei move fast enough to overcome their electrostatic repulsion and fuse together into helium, releasing tremendous bursts of energy. Nuclear fusion holds promise as a future energy source because its fuel (hydrogen) is abundant, and fusion reactions release far greater energy with less radioactive byproducts compared to nuclear fission.
Mass-Energy Equivalence
In 1905, Albert Einstein published his theory of special relativity, which revolutionized our understanding of the relationship between mass and energy. Einstein proposed that mass and energy are equivalent and can be converted into one another. This is encapsulated in his famous equation E=mc^2, where E is energy, m is mass, and c is the speed of light.
This equation shows that energy and mass are interchangeable. A small amount of mass can be converted into a tremendous amount of energy. This is because the speed of light squared is an enormous number. Even a tiny bit of matter contains an incredible amount of energy.
The implications of Einstein’s theory were not fully realized until the 1930s. Scientists discovered that the nuclei of atoms contain enormous stores of energy that could be unleashed through nuclear fission and fusion. This explained how the sun and stars shine by converting matter into energy.
The equivalence of mass and energy is one of the most important principles in modern physics. It unlocked the potential of atomic power and showed that matter is a form of condensed energy. A minute quantity of mass can release enough power to destroy cities. Einstein’s E=mc^2 equation demonstrated that mass and energy are two sides of the same coin.
Conservation Laws
Note that while mass and energy are equivalent, the total is conserved. Fusion/fission convert mass into energy without violating conservation laws. This equivalence was first proposed by Albert Einstein in 1905 with his theory of special relativity. The most well-known mass-energy equivalence formula is E=mc2, where E is energy, m is mass, and c is the speed of light. While the total energy is conserved, Einstein showed that mass can be converted into energy. A small amount of mass can produce an enormous amount of energy.
For example, nuclear fusion converts some mass into energy by fusing together light nuclei. In the process, a tiny bit of mass is lost and converted into the large amount of energy that powers the sun and other stars. Nuclear fission also converts mass into energy by splitting heavy nuclei like uranium or plutonium. In both nuclear fusion and fission, no conservation laws are broken. The total amount of mass and energy stays the same before and after the reaction by the equivalence E=mc2.
Applications of Nuclear Energy
Nuclear energy has a number of important applications in areas such as electricity generation, weapons, medicine, and spacecraft propulsion.
One of the most well-known applications of nuclear energy is for electricity generation. Nuclear power plants use controlled nuclear fission reactions to heat water and produce steam that drives turbines to generate electricity. Nuclear power provides around 10% of the world’s electricity, and some countries like France generate over 70% of their electricity from nuclear power.
Nuclear weapons utilize uncontrolled nuclear fission or nuclear fusion reactions to create explosions of tremendous destructive power. The first nuclear weapons were developed during World War 2, and nuclear arms stockpiles grew substantially during the Cold War between the US and Soviet Union. Efforts to control and reduce nuclear arsenals continue today.
Nuclear technology has many uses in medicine. Radiation is used to kill cancer cells in radiation therapy. Radioactive tracers are used in imaging techniques like PET scans to diagnose medical conditions. Nuclear medicine also uses radioactive materials for therapeutic purposes such as treating hyperthyroidism.
Spacecraft use nuclear power sources to generate electricity far from the Sun where solar panels are ineffective. Radioisotope thermoelectric generators have powered probes, landers and rovers to the outer planets and beyond. Russia has developed nuclear-powered rockets to propel spacecraft.
Safety Concerns
Nuclear energy does come with some safety concerns that need to be addressed. The most significant risks are radiation exposure, nuclear accidents, and nuclear waste.
Radiation can damage cells and cause cancer, so exposure needs to be limited. Workers at nuclear plants have dose limits and wear protection. The public also can’t be exposed to dangerous radiation levels. Plants have multiple barriers to contain radiation.
Nuclear accidents like Chernobyl and Fukushima have shown that reactor meltdowns release radiation that can harm health and the environment. Now reactors have better safeguards, but risk can’t be eliminated. Proper oversight, training, and safety culture are imperative.
Disposing nuclear waste safely is difficult due to its radioactivity. Waste is initially stored on-site in pools or dry casks. But the most radioactive waste requires permanent geologic disposal in deep underground repositories to isolate it. This is technically challenging and politically controversial.
While nuclear power does pose risks, they can be managed through good engineering, proper procedures, and oversight. With appropriate precautions and safeguards, nuclear energy’s benefits can be realized while protecting people and the environment.
Future of Nuclear Energy
The future of nuclear energy has many promising possibilities, as research continues into next-generation nuclear reactor designs as well as nuclear fusion prospects. Nuclear energy has the potential to provide abundant, carbon-free electricity to meet the world’s growing energy demands.
Several next-generation nuclear reactor designs aim to improve safety, reduce costs, and generate less waste compared to traditional reactors. These include small modular reactors, molten salt reactors, and generation IV fast neutron reactors. Small modular reactors are factory-built units that can be constructed more quickly and cheaply. Molten salt reactors use liquid fuel which improves safety. Fast neutron reactors can utilize spent fuel from traditional reactors as an energy source.
Nuclear fusion has long been considered the “holy grail” of energy research. Fusion power plants theoretically provide immense energy output with minimal radioactive waste. Fusion involves fusing lightweight atoms like hydrogen at extremely high temperatures to generate energy, similar to the process that powers stars. Major fusion research projects include ITER, a large international tokamak fusion reactor under construction in France. Smaller private fusion companies like Commonwealth Fusion Systems and TAE Technologies are also making progress on fusion reactor designs.
However, there are regulatory hurdles and public perception issues facing increased adoption of nuclear power. Concerns over nuclear waste disposal and nuclear proliferation affect nuclear power policies. Government support and safety regulations will play a key role in the extent of next-generation nuclear deployment. Overall the future looks promising for nuclear technology to supply growing electricity demand while minimizing greenhouse gas emissions, if these challenges can be properly addressed.
Conclusion
In summary, atoms absolutely can be converted into energy through nuclear physics processes like fission and fusion. This conversion is possible due to Einstein’s famous equation E=mc^2, which revealed the equivalency between mass and energy. Nuclear reactions release incredible amounts of energy from tiny amounts of matter – the fission of 1 kg of uranium can produce over 80 trillion joules of energy, millions of times more than conventional chemical reactions. This massive energy potential has enabled applications like nuclear power plants and nuclear weapons, which have had profound and controversial impacts on society. Nuclear physics remains an area of active research, with much still to be learned about safely harnessing the immense power within the atom. But the core discovery that matter and energy are interchangeable has forever changed our understanding of the universe.