What Is The Spectra Produced By The Sunlight?
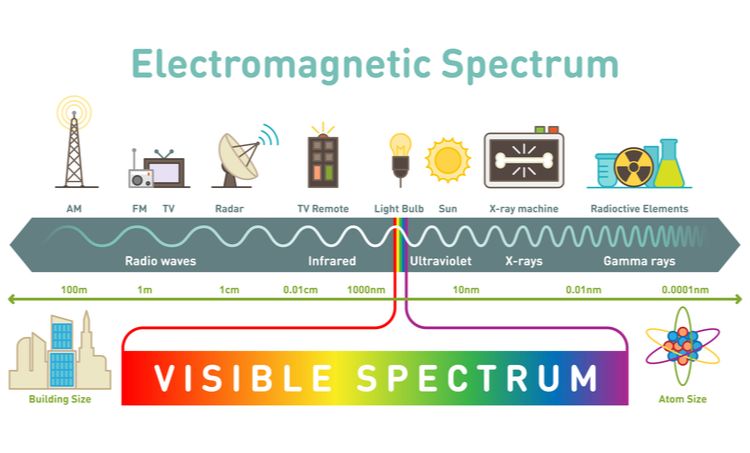
The electromagnetic spectrum is the full range of electromagnetic radiation that exists. This includes all the frequencies and wavelengths of electromagnetic energy. Though we commonly think of visible light when we think about the spectrum, visible light makes up only a small portion of the full electromagnetic spectrum.
Electromagnetic radiation can be described in terms of a stream of photons, which are packets of energy, or in terms of waves, which are oscillating electric and magnetic fields. Wavelength and frequency are inversely related – as wavelength increases, frequency decreases. The spectrum is generally divided into specific regions based on the frequency and wavelength, including (from long to short wavelengths) radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, and gamma rays.
The electromagnetic spectrum originates from various natural and artificial processes, including the sun. The differing wavelengths interact with matter in unique ways, which enables a wide variety of technologies and sciences across the full spectrum.
Visible Light Spectrum
The visible light spectrum is the range of wavelengths of electromagnetic radiation that the human eye can see. It is the part of the full electromagnetic spectrum that is visible to the human eye.
The visible spectrum runs from approximately 380 nanometers to 750 nanometers. It consists of the colors humans are able to perceive, from violet and blue at the short wavelength end, through greens and yellows, to orange and reds at the long wavelength end. The colors can be represented by the acronym ROYGBIV, which stands for:
- Red
- Orange
- Yellow
- Green
- Blue
- Indigo
- Violet
These colors make up the rainbow spectrum Newton first identified when he passed sunlight through a prism. The visible spectrum contains all the color wavelengths that the human eye can detect and distinguish between.
Infrared Spectrum
Infrared waves, while invisible to human eyes, play an important role in transmitting heat. Objects emit infrared radiation as a function of their temperature. The hotter an object, the more infrared radiation it emits. Infrared waves have longer wavelengths than visible light, generally measuring from 700 nanometers to 1 millimeter. This longer wavelength means infrared waves have lower frequency than visible light.
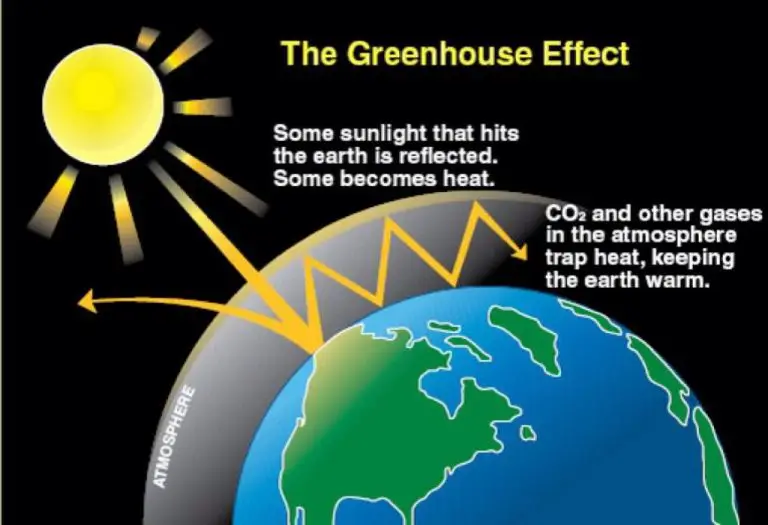
Infrared waves are sometimes called heat waves, because they are emitted by hot objects and detected as heat by our skin. However, all electromagnetic waves exhibit the same heat effects if absorbed at a high enough intensity. Infrared radiation from the Sun accounts for 49% of the heating of Earth. Greenhouse gases like carbon dioxide and methane are able to absorb infrared radiation, contributing to global warming through the greenhouse effect.
Night vision technologies make use of infrared’s ability to penetrate darkness. Special cameras detect infrared radiation emitted by warm objects against cooler backgrounds. This allows important applications such as night vision, thermal imaging, and remote temperature sensing. For example, infrared cameras can detect heat loss in homes and help diagnose circulation problems in the human body.
Ultraviolet Spectrum
The ultraviolet (UV) spectrum is just beyond the violet end of the visible light spectrum. UV light has shorter wavelengths and higher frequencies than visible light. The wavelengths of UV light range from 10 nanometers (nm) to 400 nm.
UV light has several unique properties. It contains more energy per photon than visible light, which allows it to cause chemical reactions and damage materials. This is why overexposure to UV rays can be harmful to human health. At the same time, the energy in UV light also allows it to disinfect surfaces.
In terms of health effects, UV light is categorized into UV-A, UV-B, and UV-C bands. Prolonged exposure to UV-B and UV-C radiation can cause sunburn and skin cancer. Meanwhile, UV-A rays penetrate deeper and can cause premature aging and wrinkles. The ozone layer in the upper atmosphere blocks most UV-B and virtually all UV-C from reaching the earth’s surface. Without the protection of the ozone layer, these UV rays would be very damaging to life.
UV light has both risks and benefits. While overexposure is harmful, moderate exposure enables the human body to produce vitamin D. Engineers have also harnessed UV in various sterilization applications. The unique properties of ultraviolet light have allowed it to play an important role across many different domains.
Radio Waves
Radio waves are one type of electromagnetic radiation produced by the sun. They have wavelengths longer than infrared light. Radio waves have frequencies from 3Hz to 300 GHz, which corresponds to wavelengths between 100 km and 1 mm. Some key properties of radio waves:
- At the low frequency end, radio waves can penetrate thousands of kilometers into space and Earth’s atmosphere.
- Higher frequency radio waves, like microwaves, are absorbed by atmospheric gases.
- Radio waves are widely used for communication technologies like radio, television broadcasts, WiFi, and mobile phones.
- Different frequencies of radio waves have different propagation properties useful for different applications.
- Lower frequency waves can diffract around large obstacles and travel beyond the horizon, while higher frequencies travel in straighter paths.
The most familiar uses of radio waves are AM/FM audio broadcasts. AM (amplitude modulation) involves varying the amplitude of radio waves to transmit audio signals. FM (frequency modulation) varies the frequency slightly to encode audio. AM travels farther than FM, but FM has higher audio fidelity.
Microwaves
Microwaves are a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter, or frequencies between 300 MHz and 300 GHz. Some key properties of microwaves include:
Microwaves have the ability to heat water, fats, and sugar molecules in food. When food molecules absorb microwave energy, they begin vibrating rapidly, creating friction and heat. This property allows microwave ovens to cook food very quickly. Microwave heating is used in many industrial and scientific applications as well.
Microwaves are commonly used for wireless communication, including radio broadcasting, radar, satellite communication, and wireless networking. For example, WiFi routers transmit and receive microwaves to enable wireless internet connectivity.
Microwaves are used in microwave spectroscopy to study the structure of molecules by analyzing how they interact with and absorb microwave radiation.
In astronomy, microwaves emitted by cosmic microwave background radiation and from the gas and dust between stars and galaxies are studied to understand the history and structure of the universe.
X-Rays
X-rays are a form of high-energy electromagnetic radiation with wavelengths that range from about 10 nanometers to 10 picometers. X-rays have extremely short wavelengths compared to visible light, which allows them to penetrate most materials and make internal images of the body and other objects possible. X-rays were first discovered by German physicist Wilhelm Röntgen in 1895 and are commonly used today for medical imaging and security screening applications.
In medicine, X-rays are used in a variety of imaging techniques like X-ray radiography, CT scans, and mammography to view bone fractures, tumors, dental issues, and other abnormalities inside the body. The high energy of X-rays allows them to pass through soft tissues and reveal problems with dense structures like bones. However, X-ray radiation does carry potential health hazards at high doses including an increased cancer risk, so safe practices are important. Medical imaging technicians take precautions like shielding and dosage limits to avoid overexposing patients.
In addition to medicine, X-ray scanners are also important security tools at airports, government buildings, and other sensitive sites to detect weapons, explosives, and contraband hidden in luggage or on a person’s body. The ability for X-rays to penetrate materials makes them ideal for seeing inside objects without having to open or dismantle them. Advanced imaging algorithms can identify suspicious and banned items automatically. However, privacy concerns exist around the revealing nature of backscatter X-ray scans of people.
Gamma Rays
Gamma rays are the most energetic form of electromagnetic radiation, with extremely short wavelengths and very high frequencies. They are produced by some of the most violent events in the universe, such as supernovae, neutron stars, and black holes.
On Earth, gamma rays do not penetrate the lower atmosphere well, but they can be produced by nuclear explosions, lightning strikes, and radioactive decay from unstable atomic nuclei. Gamma rays have high penetrating power and can pass through most materials, requiring heavy shielding like lead or concrete to block them.
Due to their high energy and ionizing capability, prolonged exposure to gamma radiation is hazardous to living tissues. However, gamma rays have important medical applications in radiation treatments to kill cancer cells. They also enable detailed imaging for nuclear medicine and security screening.
In astronomy, gamma ray bursts are among the most energetic events observed in the universe. These bursts are intense explosions associated with the collapse of massive stars or collisions between neutron stars. Gamma rays enable the study of high-energy astrophysical processes that produce cosmic rays.
Creating the Spectrum
The sun produces a wide spectrum of electromagnetic radiation due to the extremely high temperatures and pressures present in its core. At the sun’s core, hydrogen atoms fuse together in a process called nuclear fusion, releasing enormous amounts of energy. This energy radiates outwards through the different layers of the sun in the form of photons, or particles of light.
As these photons travel outward, they interact with the material in the different layers of the sun through various absorption and emission processes. Lower energy photons can pass through freely, emerging as visible light and lower energy infrared and radio waves. Higher energy photons get absorbed and re-emitted multiple times, eventually emerging as ultraviolet light and even higher energy x-rays and gamma rays.
The density and temperature of the material determines which wavelengths get absorbed or emitted. This filtering process combined with the wide spectrum of photons produced by nuclear fusion leads to the continuous spectrum of electromagnetic radiation emitted by the sun across radio, microwave, infrared, visible light, ultraviolet, x-ray and gamma ray frequencies.
Importance to Life
The various wavelengths of light that make up the electromagnetic spectrum emitted by the Sun have significant impacts on life on Earth. The visible light enables plants to photosynthesize and produce energy. The ultraviolet rays can damage DNA and cause sunburns, but are also needed in moderation by the body to produce vitamin D. Infrared radiation is felt as heat and helps regulate Earth’s temperature. Radio waves are harnessed for communication technologies like radio, television, and WiFi.
At the higher energy end, x-rays and gamma rays can ionize atoms and cause radiation damage. But x-rays are used carefully in medicine and gamma rays spark mutations that drive evolution. Overall, life has evolved to both utilize the beneficial wavelengths of the solar spectrum and protect itself from the more harmful ones. The different parts of the spectrum power essential biological, atmospheric, and technological processes.