Why Is Hydrogen Energy Bad For The Environment?
Hydrogen is considered a clean fuel and a potential solution for reducing greenhouse gas emissions and mitigating climate change. When used in a fuel cell, hydrogen combines with oxygen to produce electricity, with water as the only byproduct. Unlike fossil fuels, hydrogen does not emit carbon dioxide or other air pollutants when used. For these reasons, hydrogen is often touted as an environmentally friendly energy source. However, producing, transporting, storing, and using hydrogen does have some environmental impacts that should be considered. This article will evaluate the various ways hydrogen can affect the environment, in order to provide a balanced perspective on hydrogen as a clean fuel source.
Production Methods
Hydrogen is mainly produced through two methods – electrolysis using electricity to split water into hydrogen and oxygen, and steam reforming of natural gas. Currently over 95% of hydrogen is produced from fossil fuels via steam reforming, coal gasification or partial oxidation of methane and oil.[1] This process uses high temperature steam to split hydrogen from carbon atoms in natural gas. The other 5% is produced via electrolysis using electricity, most commonly derived from fossil fuels like coal or natural gas.[2]
Because the predominant method of hydrogen production relies heavily on fossil fuels, it is not a fully renewable or clean energy source. Extracting hydrogen from methane releases carbon dioxide, contributing to greenhouse gas emissions and global warming. Even electrolysis relies on electricity that is mostly generated by fossil fuels currently. The high fossil fuel dependence means hydrogen production is not yet sustainable or environmentally friendly.[3]
Fossil Fuel Reliance
Most hydrogen produced today comes from fossil fuels like natural gas and coal, which results in greenhouse gas emissions. According to the The Role of Carbon Capture and Storage book, over 20% of hydrogen comes from reforming natural gas. The process of steam reforming natural gas releases carbon dioxide, a major greenhouse gas. Coal gasification is another common method for hydrogen production that also emits CO2.
According to the Global Witness report, 95% of hydrogen today is produced from fossil fuels. This heavy reliance means most hydrogen production currently contributes to climate change rather than reduces emissions. Significant development of renewable hydrogen production methods is needed for hydrogen to be a truly clean energy source.
Water Usage
One of the main environmental concerns around hydrogen production is the large amount of water required, especially when using electrolysis. Electrolysis uses an electric current to split water (H2O) into hydrogen and oxygen. To produce 1 kg of hydrogen via electrolysis requires around 9 kg of water (1). At large scale, this adds up to significant water usage. For example, a 100 ton per day “green” hydrogen production facility in Utah is projected to consume 730,000 cubic meters of water per year (2).
However, studies show that water usage for a global hydrogen economy would be relatively small compared to total water usage. One analysis estimates that producing enough “green” hydrogen to meet global energy demands in 2050 would require around 25 billion cubic meters of water. While substantial, this represents less than 1% of total global water withdrawals (3).
Overall, hydrogen production does require large volumes of water, especially via electrolysis. But studies suggest this can be managed, and some methods like steam methane reforming use far less water. Strategic siting of production, usage of non-potable water sources, and recycling water can also help minimize impacts.
(1) https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis
(2) https://rmi.org/hydrogen-reality-check-distilling-green-hydrogens-water-consumption/
(3) https://energypost.eu/hydrogen-production-in-2050-how-much-water-will-74ej-need/
Renewable Options
Hydrogen can be produced from renewable sources like biomass or solar and wind power, providing a sustainable alternative to fossil fuel-based production methods. However, renewable hydrogen production is currently limited in scalability and costly compared to conventional methods.
Biomass gasification is one renewable method for producing hydrogen. Through a thermal process involving heat, steam, and oxygen, biomass feedstocks like wood chips or agricultural waste can be converted into hydrogen and other byproducts, without combustion [1]. Although promising, biomass gasification facilities require significant capital investment and abundant biomass resources to produce hydrogen at scale.
Electrolysis powered by solar or wind energy can split water into hydrogen and oxygen in a clean, renewable process. But building enough solar panels or wind turbines to produce sufficient electricity for major hydrogen production remains prohibitively expensive. Renewable electrolysis may be better suited for distributed or off-grid hydrogen applications currently [2].
While renewable hydrogen production methods are advancing, most experts agree fossil fuel-based steam methane reforming will continue meeting large-scale hydrogen demand in the near future.
Storage Difficulties
Hydrogen has an extremely low density, which makes it difficult to store in large quantities. According to the U.S. Department of Energy, “High density hydrogen storage is a challenge for stationary and portable applications and remains a significant challenge for transportation applications” (https://www.energy.gov/eere/fuelcells/hydrogen-storage).
Because hydrogen is so light and takes up so much space, it requires high-pressure tanks or cryogenic cooling to become dense enough for storage and transportation. This leads to energy loss through compression and liquefaction. According to a 2023 study, “There are many associated problems such as weight, volume, weight-to-volume ratio of the storage system, and most important of all, Hydrogen Embrittlement” (https://www.sciencedirect.com/science/article/abs/pii/S036031992300530X). The energy spent compressing or cooling hydrogen can represent a significant efficiency loss.
Improving hydrogen storage technology remains an active area of research. But currently, the difficulties of storing lightweight hydrogen gas pose a challenge for widespread hydrogen adoption. Compression, liquefaction and advanced storage materials may help address these issues in the future.
Transportation Issues
Transporting hydrogen is a major challenge, as hydrogen gas has very low density. To achieve sufficient energy density for practical transportation, hydrogen needs to be compressed or liquefied. According to the U.S. Department of Energy, “High density hydrogen storage is a challenge for stationary and portable applications and remains a significant challenge for transportation applications.”
Compressing hydrogen to high pressures requires a significant amount of energy. Estimates are around 6.0 kWh/kg for compression to 70 MPa. As noted in a study by Argonne National Laboratory, “The compression of hydrogen is an energy-intensive process that increases the overall cost.” (Sciencedirect)
Liquefying hydrogen into a cryogenic liquid also consumes substantial energy, around 30% of the energy content of the hydrogen. Due to hydrogen’s low boiling point, maintaining it as a liquid requires constant cooling and insulation. This makes storage and transportation as a liquid quite inefficient.
Overall, the need for compression or liquefaction adds complexity and energy usage for transporting hydrogen. This reduces the net environmental benefits of hydrogen versus other energy carriers that can be more easily transported with less processing, such as electricity or liquid fuels.
Safety Risks
Hydrogen poses significant safety risks due to its high flammability and explosiveness. Hydrogen has a very wide flammability range of 4-75% in air, compared to gasoline which has a narrow flammability range of 1.4-7.6% (https://en.wikipedia.org/wiki/Hydrogen_safety). This makes hydrogen leaks and accidents much more likely to result in ignition and explosion. Hydrogen flames are nearly invisible, requiring thermal imaging cameras to detect them. Hydrogen is also odorless, colorless, and tasteless, making leaks difficult to detect.
The primary risk with hydrogen is the potential for BLEVE – boiling liquid expanding vapor explosion (https://en.wikipedia.org/wiki/Hydrogen_safety). This can occur when cryogenic liquid hydrogen is released and rapidly vaporizes, resulting in an explosion. Hydrogen storage and transportation often utilizes liquefied hydrogen at extremely cold temperatures, increasing the risk of BLEVEs.
There have been numerous hydrogen explosion incidents at refueling stations and hydrogen production facilities. In 2019, a hydrogen tube trailer exploded at an ExxonMobil refinery in California, injuring 4 workers (https://www.hazardexonthenet.net/article/199696/Recent-developments-in-the-field-of-hydrogen-safety.aspx). In June 2022, a hydrogen plant explosion in Wenzhou, China killed 2 people and injured 12 (https://www.hazardexonthenet.net/article/199696/Recent-developments-in-the-field-of-hydrogen-safety.aspx). These incidents highlight the very real dangers posed by hydrogen’s high flammability and explosiveness.
Other Environmental Impacts
Hydrogen infrastructure like pipelines can damage ecosystems and wildlife habitats. Constructing pipelines requires clearing land which fragments habitats. This disrupts migration patterns and breeding habits for animals. Pipelines also risk contamination. Leaks and ruptures could pollute soils, groundwater, and aquatic ecosystems. Fugitive emissions from hydrogen pipelines contribute to air pollution. The small size of hydrogen molecules increases leakage risks. According to EDF, around 0.12% of hydrogen transported in pipelines leaks into the atmosphere daily. This worsens air quality and public health. Hydrogen piping materials like steel are also vulnerable to hydrogen embrittlement over time. This increases the chances of leaks and explosions.
Conclusion
In summary, there are several key reasons why hydrogen is currently detrimental to the environment:
Most hydrogen production relies on fossil fuels, which emit greenhouse gases. Extracting hydrogen from natural gas or coal continues our dependence on nonrenewable resources. While hydrogen itself may be clean, the methods to produce it largely aren’t yet.
Generating hydrogen uses substantial amounts of water, which strains local water resources. This could become problematic in areas already experiencing droughts.
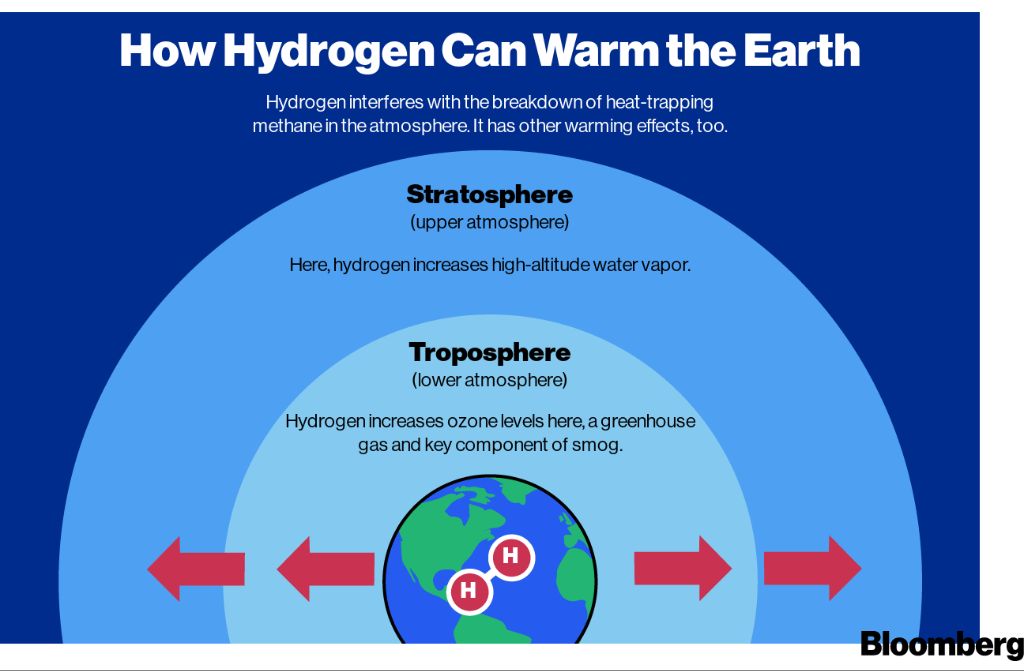
Storing and transporting hydrogen safely and efficiently remains a challenge. Potential hydrogen leaks contribute to global warming and ozone depletion. The lack of hydrogen infrastructure also inhibits adoption.
However, with further development of renewable hydrogen production from solar, wind, or nuclear energy, hydrogen could provide a crucial zero-emission fuel source. Advances in storage and distribution infrastructure could also enable a future hydrogen economy. But currently, widespread hydrogen use may replace one environmental harm with another. With improved technology and practices, hydrogen may someday live up to its clean energy promise.
The key takeaway is that while hydrogen has environmental drawbacks in its present form, innovation and sustainable practices could pave the way for greener hydrogen energy. But as of now, hydrogen risks causing new environmental issues without yet solving old ones.